Decades of Cancer Vaccine Research Enabled Rapid Development of COVID-19 Vaccines
Editor’s note: This post was written by Nicholas Warren, PhD, science policy program administrator in the AACR’s Office of Science Policy and Government Affairs. Warren was a contributing author of the Clinical Cancer Research paper discussed in the post.
This December, the U.S. Food and Drug Administration (FDA) authorized two RNA-based vaccines to prevent infection with SARS-CoV-2, the virus that causes COVID-19. The rapid development of these vaccines was made possible through an unprecedented level of global scientific collaboration, robust clinical trial participation from tens of thousands of volunteers, and decades of strong federal support of previous research on related viruses and cancer.
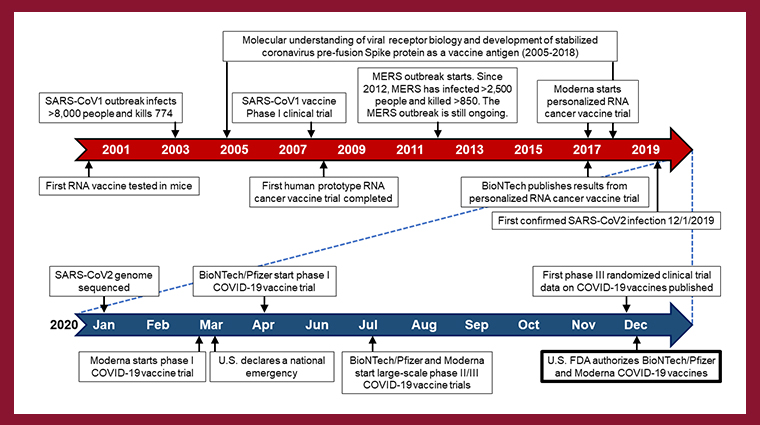
A new Clinical Cancer Research article by the American Association for Cancer Research’s (AACR) COVID-19 and Cancer Task Force describes how the COVID-19 vaccines made by BioNTech-Pfizer and by Moderna are based on the companies’ previous research into personalized therapeutic RNA cancer vaccines. These vaccines have been in development for decades, with the first RNA vaccine being tested in mice in the year 2000 (see figure below). These vaccines function by entering cells, which then use the RNA as a guide to build proteins that the immune system recognizes as dangerous. The vaccines’ RNA is gradually degraded and recycled by enzymes inside the body. To date, no RNA cancer vaccines have been approved, but eight pharmaceutical companies are studying them in clinical trials.
With this research as a platform, BioNTech-Pfizer and Moderna were able to generate the first COVID-19 vaccines and start phase I clinical trials roughly two months after the genome sequence of SARS-CoV-2 was published. For comparison, it took more than nine months to start a phase I clinical trial for an Ebola vaccine during the 2014 outbreak.
Antoni Ribas, MD, PhD, FAACR, AACR President and author of the Clinical Cancer Research article, said, “These remarkable vaccines demonstrate the importance of decades of investment in basic science and cancer research. It is critical that we inform patients with cancer about the importance of COVID-19 vaccination to prevent them from contracting the virus so we can safely treat their cancer.”
E. John Wherry, PhD, member of the AACR’s COVID-19 and Cancer Taskforce and lead author of the Clinical Cancer Research article, said, “I have never seen such robust efficacy and safety profiles from a vaccine on the first try, and we have two of them. Hopefully this technology can be used to develop vaccines for other deadly diseases.”
The AACR is committed to helping patients with cancer during this pandemic through timely communication of important developments. As part of this commitment, all AACR journal publications related to the COVID-19 pandemic are freely available on the COVID-19 and Cancer Resource Center. Additionally, the AACR Scientist↔Survivor Program, led by Anna D. Barker, PhD, hosts virtual Patient Advocate Forums to help share information about the pandemic and latest advances in science. We also encourage patients to discuss specific concerns about vaccines with their doctors.