AACR Journal Editors Share “Must Read” Articles for February
Back for the month of February is our regular staple, Editors’ Picks, which features the “must read” articles that are selected by the editors of the AACR scientific journals. In this edition, featured studies include results from two clinical trials, the characterization of a new ERK1/2 inhibitor, and a comparison of different oils used for intraperitoneal application in mouse models, among other studies. What’s more, February is National Cancer Prevention Month, and two studies from this month’s selections highlight at-home screening approaches for the early detection of cancer. As always, articles featured here are freely available for a limited time.
Journal: Molecular Cancer Therapeutics
The Ras signaling pathway, which mediates key cellular processes such as differentiation, motility, and apoptosis, is frequently dysregulated in cancer and has therefore been investigated as a potential target for anticancer treatment for decades. Single-agent inhibitors of this pathway have shown limited clinical success, and much research is focused on investigating the combinatorial effects of inhibiting multiple members of the Ras signaling cascade. In this study, the authors describe the discovery and characterization of AZD0364, an inhibitor of ERK1/2, a protein downstream of Ras. In vitro and in vivo experiments in BRAF- and KRAS-mutant cell lines and xenograft models revealed that AZD0364 treatment resulted in reduced proliferation and led to tumor regression, respectively. Further, dual inhibition of ERK1/2 with AZD0364 and MEK1/2 (another protein downstream of Ras) with selumetinib (Koselugo) resulted in synergistic suppression of the Ras signaling pathway in KRAS-mutant cell lines and resulted in significant tumor regressions in multiple KRAS-mutant xenograft models. The authors conclude that the combination of ERK1/2 and MEK1/2 inhibition may be a viable strategy for the treatment of KRAS-mutant malignancies. This article was highlighted in the February issue.
Journal: Cancer Immunology Research
Inhibition of the BRAF and/or MEK proteins is a standard-of-care therapy for BRAF-mutated melanoma; however, these inhibitors can impact the efficacy of immune checkpoint inhibitors. Inhibition of CDK4/6 kinases has been shown to improve the efficacy of BRAF and MEF inhibition, but the impact of this triple therapy on antitumor immunity remains unclear. Utilizing a BRAF/CDK-mutated melanoma mouse model, the authors demonstrated that triple therapy with BRAF, MEK, and CDK4/6 inhibitors led to reduced numbers of proinflammatory macrophages and cross-priming CD103+ dendritic cells in the tumor microenvironment. Isolated immune cell populations from mice treated with triple therapy failed to induce T-cell responses in ex vivo experiments. Furthermore, the absence of proinflammatory macrophages and cross-priming CD103+ dendritic cells in patient tumors was associated with poor clinical responses to immune checkpoint blockade and poor overall survival. Together, the results indicate that triple therapy may have immunomodulatory effects that could negatively impact responses to immune checkpoint blockade. This article was featured on the cover of the February issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Offering self-collection of cervical samples for human papillomavirus (HPV) testing may increase cervical cancer screening rates but may be approximately 2 percent less effective than clinician-collected samples. In this study, the authors modeled how exclusive use of self-collection may impact lifetime cervical cancer incidence and mortality in Australia. Among women who are not vaccinated against HPV, the benefits of self-collection screening are likely to offset the potential loss of sensitivity if at least 15 percent of unvaccinated women participate. Self-collection screening would be expected to reduce cancer cases and deaths by 10-18.5 percent if 50 percent of unvaccinated women participated and by 17.6-31.2 percent if 80 percent of unvaccinated women participated. For women who are vaccinated, 50 percent uptake of self-collection screening would reduce cancer cases and deaths by 6.2-12.4 percent and by 12.2-23 percent if 80 percent of vaccinated women participated. The authors conclude that the benefits of self-collection for cervical cancer screening outweigh potential risks associated with reduced testing sensitivity. This article was highlighted in the February issue, and a related commentary can be found here.
Journal: Cancer Research (February 15 issue)
Among patients with myelodysplastic syndromes and acute myeloid leukemia (AML), those who have adverse genetic features (e.g., monosomal karyotypes, such as losses on chromosome 5, 7, or 17) have been reported to respond favorably to treatment with hypomethylating agents such as decitabine (Dacogen) or azacitidine (Vidaza). However, the underlying reason for this observed efficacy in this patient population is not well understood. Using RNA-Seq, the authors evaluated AML cell lines with or without a chromosome deletion (5q, 7q, or 17p) that were treated with the hypomethylating agent decitabine. They found that treatment with decitabine resulted in the preferential upregulation of several tumor suppressive genes when they were in a hemizygous state. Treatment with decitabine also derepressed the ERV3-1 (for endogenous retrovirus group 3 member 1) gene and activated the dsRNA sensor RIG-I. These results were validated by mapping in vivo transcriptional changes in primary blood blast cells isolated from patients with AML receiving decitabine. Further, compared with a structurally similar chemotherapeutic agent that lacks hypomethylating activity (cytarabine), treatment with decitabine resulted in superior survival rates in patient-derived murine monosomal karyotype AML xenografts. The authors conclude that these results help to unravel the intriguing clinical activity of hypomethylating agents among patients with AML with chromosome 7 deletions and other karyotypes. This article was highlighted in the February 15 issue. A related commentary can be found here.
Journal: Cancer Research (February 1 issue)
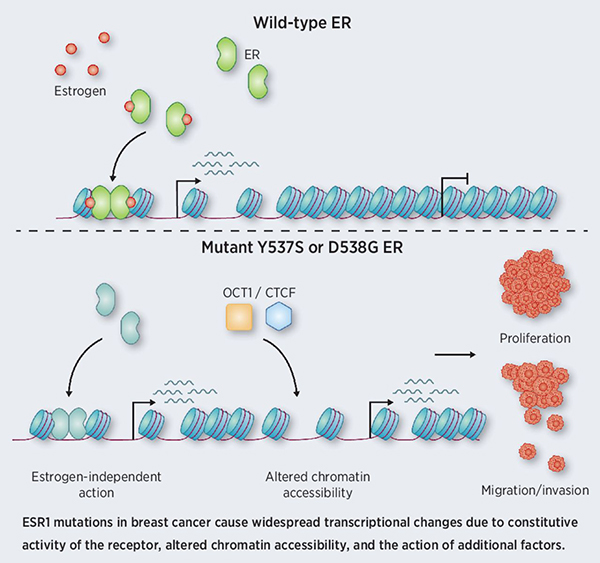
Approximately 30 percent of estrogen receptor (ER)-positive breast cancers that relapse after hormone therapy have mutations in the segment of the ESR1 gene that encodes the ER ligand binding domain (LBD). These mutations render the ER constitutively active, even in the absence of estrogen. The consequences of such mutations remain unclear. Here, the authors developed isogenic breast cancer cell lines for the two most common LBD mutations, Y537S and D538G, to investigate their impacts. They found that each of these mutations led to gene expression changes for thousands of genes. Many of these gene expression changes were not observed when cells with wild-type ER were treated with estrogen, indicating that some of the gene expression changes observed with mutated ER were independent of overactive ER signaling. The researchers also observed substantial differences in chromatin accessibility in cells with mutated ER. Several DNA-binding factors, including FOXA1, CTCF, and OCT1, were associated with mutant-enriched accessible chromatin regions. The authors conclude that LBD-mutated ER induces widespread changes in gene expression through upregulation of ER signaling and changes to chromatin accessibility. A related commentary can be found here.
Journal: Molecular Cancer Research
In animal experiments, oils are frequently used to dissolve lipophilic substances (such as tamoxifen for inducible gene recombination). The oil solution is then delivered to the animal either through oral gavage or through intraperitoneal injection. While oils have been shown to induce inflammation following peritoneal injection, comparative studies analyzing the inflammatory effects induced by different oils are lacking. In this study, the authors evaluated the effects of injecting olive, peanut, corn, and mineral oil into the peritoneum of mice. While all four oils induced inflammation to some degree, the severity and duration of the inflammation were different for each oil, which, the authors note, has the potential to affect subsequent experimental outcomes. The authors also compared how peritoneal injection of peanut oil compared with oral gavage of peanut oil in an inducible peritonitis murine model. They found that the changes in the myeloid cell composition in the peritoneum of the injected animals resulted in impaired resolution of the induced inflammation, and advise that lipophilic substances should be delivered by oral gavage instead of intraperitoneal injection. The authors also note that this work contributes to improving the reproducibility of animal research, as the selection of different oils resulted in different inflammatory effects in their mice. This article was highlighted and featured on the cover of the February issue.
Journal: Blood Cancer Discovery
Changes in DNA methylation patterns have been observed in cancer cells, but the timing of this aberrant methylation remains unclear. In this study, the authors used bisulfite sequencing to characterize methylation patterns in patient samples of preneoplastic monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL) before disease onset and throughout disease progression and treatment. They found that aberrant methylation patterns were already present in CLL at diagnosis and that methylation patterns did not change dramatically during the MBL-to-CLL transition. Methylation patterns remained stable throughout disease progression and therapy. The authors conclude that aberrant methylation associated with cancer occurs early and propose that changes in DNA methylation may play a central role in disease development. A related commentary can be found here.
Journal: Clinical Cancer Research (February 1 issue)
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, and recent clinical trials evaluating checkpoint inhibitors have revealed promising results among patients with advanced HCC. In this phase II clinical trial, the PD-L1 inhibitor avelumab (Bavencio) was evaluated among 30 patients with advanced HCC who were previously treated with the anti-angiogenesis agent sorafenib (Nexavar). After a median follow-up of 13.9 months, three patients (10 percent) experienced a partial response and 19 patients (63.3 percent) experienced stable disease. The overall response rate (ORR) was 10 percent; the study did not reach its primary endpoint (ORR of 15 percent). Patients with a longer duration of prior sorafenib treatment had better outcomes following treatment with avelumab, and PD-L1 expression did not affect avelumab responses. The authors note that avelumab treatment may be an option for patients with sorafenib-refractory advanced HCC. This article was highlighted in the February 1 issue.
Journal: Clinical Cancer Research (February 15 issue)
Balancing therapeutic efficacy with treatment-related toxicity is a key factor in the development of an anticancer regimen. Systemic treatments for triple-negative breast cancer (TNBC) typically revolve around chemotherapy, and the optimal chemotherapeutic regimen for the treatment of this aggressive disease is still being explored. This study reports on results from the NeoSTOP clinical trial, which compared two carboplatin-containing neoadjuvant chemotherapy regimens (one that contains an anthracycline, and one that does not) among 100 patients with stage 1-3 TNBC. After a median follow-up of 38 months, the event-free survival and overall survival were similar between both regimens, yet grade 3-4 adverse events were more common among patients treated with anthracycline-containing treatment. Further, the cost of treatment was significantly lower among patients not receiving anthracycline. The authors conclude that the non-anthracycline-containing regimen was cheaper and less toxic than the anthracycline-containing regimen, but had similar clinical efficacy outcomes. This article was highlighted in the February 15 issue.
Journal: Cancer Discovery
Selection of Oncogenic Mutant Clones in Normal Human Skin Varies with Body Site

The risk of skin cancer varies between different regions of the body, with more exposed parts of the body being at higher risk due to increased exposure to ultraviolent (UV) light. In this study, the authors sequenced normal skin cells to characterize the genetic mutations that are present in various parts of the body. They found that the density of mutations varied, with higher mutation densities observed in skin cells from regions with greater UV exposure and higher skin cancer risk. While skin cells from across the body showed evidence of UV-induced mutations, mutational signature analysis revealed differences in DNA repair processes between skin cells from different regions of the body. Their analysis also showed that 11 mutant genes were under positive selection in various parts of the body, including TP53 and FAT1 in skin cells from the head and leg, respectively. However, mutations in the NOTCH1 and FAT1 genes were found in normal skin cells of the forearm, trunk, and leg at similar densities to those observed in skin cancers, raising the possibility that mutations in these genes may not be drivers of tumorigenesis. Analysis of mutations in the hair follicles showed differences between the upper follicle and lower follicle, with mutations in the upper follicle more closely resembling those of the adjacent skin and the lower follicle remaining highly polyclonal with few mutations. The authors conclude that the mutations in normal skin cells vary widely across different parts of the body. This article is highlighted and featured on the cover of the February issue. A related commentary can be found here.
Journal: Cancer Prevention Research
Although colorectal cancer is the second-most prevalent cancer in Brazil, there is no nationally implemented screening program. This article reports results from a fecal immunochemical test (FIT)-based screening program implemented at Barretos Cancer Hospital, which is one of Brazil’s largest medical institutions and serves underserved populations. Between 2015 and 2017, 6,737 eligible patients in the outpatient department who were between 50 and 65 years old were invited to participate. Among those invited, 92.8 percent completed and returned their FIT, and 12.5 percent of these patients had a positive FIT result. Colonoscopy compliance for those referred was 84.6 percent. Positive predictive values of the FIT for adenoma, advanced adenoma, and colorectal cancer were 60 percent, 16.5 percent, and 5.6 percent, respectively. Among the patients diagnosed with cancer, diagnoses from the screening program occurred at earlier cancer stages than clinically diagnosed cancers reported in Barretos Cancer Hospital and in Brazil cancer registries. The authors suggest that implementation of a screening program could improve outcomes from colorectal cancer in Brazil. This study was featured on the cover of the February issue.