Editors’ Picks: October Highlights from AACR Journals
Among the studies published during the month of October in the nine AACR journals, the editors have chosen to highlight results from two clinical trials of DNA damage response inhibitors; a study describing how red meat consumption may cause DNA damage that leads to cancer-causing mutations and promotes colorectal cancer development; and a potential vaccine strategy for pancreatic cancer, among others. Enjoy reading our summaries and check out the highlighted articles, which are freely available online for a limited time.
Journal: Blood Cancer Discovery
Overcoming Plasma Protein Inhibition of Tyrosine Kinase Inhibitors
FMS-like tyrosine kinase 3 (FLT3) is the most commonly mutated gene in acute myeloid leukemias. Tyrosine kinase inhibitors (TKIs) targeting FLT3 have had limited success in treating AML, in part due to the suppression of TKI activity by patient-specific factors, such as plasma proteins that bind to the TKI. In this study, the researchers examined the impact of the human alpha-1-acid glycoprotein (AGP), a plasma protein, on the efficacy of a staurosporine-derived TKI (STS-TKI). They found that human AGP inhibited the activity of STS-TKI in FLT3-mutated cells, whereas treatment of cells with bovine plasma or bovine AGP did not inhibit STS-TKI activity. Cotreatment of cells with human AGP and the AGP-binding drug mifepristone improved the efficacy of STS-TKI, suggesting that mifepristone’s binding to AGP may prevent its inhibition of STS-TKI. In a mouse model of AGP drug inhibition, mifepristone restored the activity of the FLT3-targeted TKI midostaurin (Rydapt). Together, the results suggest that the limited clinical efficacy of FLT3-targeted TKIs in AML may be due to inhibitory binding by patient AGP. The authors propose cotreatment with mifepristone may improve the efficacy of FLT3-targeted TKIs in patients. This article is highlighted in the October issue.
Journal: Molecular Cancer Therapeutics
The mitogen-activated protein kinase (MAPK) pathway is one of the main signaling cascades that stimulate cell proliferation. This pathway is aberrantly activated in approximately 40 percent of human cancers, and there are several drugs that target different components of this pathway. Despite their clinical effectiveness in the short term, resistance is common and often due to reactivation of ERK kinase, the primary effector of the MAPK pathway. This study reports on the highly potent and selective ERK inhibitor ASTX029, which functions through a dual mechanism blocking both the protein’s enzymatic activity and its phosphorylation by MEK kinase, though it does not directly inhibit MEK. The authors showed that ASTX029 inhibits the proliferation of MAPK-activated cell lines, including those carrying BRAF and RAS mutations, and has antitumor activity in vivo in xenograft models. They also demonstrated activity in both in vitro and in vivo models against acquired resistance to other MAPK pathway inhibitors. ASTX029 is currently being evaluated in the clinic in a phase 1-2 trial in patients with advanced solid tumors. This article is highlighted in the same issue.
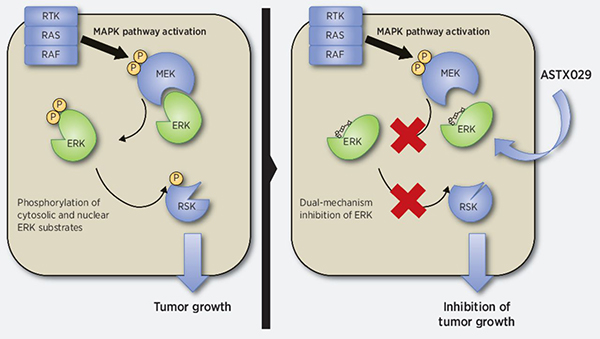
Journal: Clinical Cancer Research (October 1 issue)
The accumulation of DNA damage is a common feature of cancer cells, which rely on DNA repair mechanisms to prevent severe genomic instability and subsequent cell death. The kinase ATR is a crucial component of the DNA double-stranded break repair pathway, which has made it an attractive target for new cancer drugs. Ceralasertib, a selective inhibitor of ATR, has been shown in preclinical studies to synergize with chemotherapies such as carboplatin. In this phase I clinical trial, the researchers treated 36 patients, with various solid tumors, with a fixed dose of carboplatin plus escalating doses of ceralasertib. Carboplatin was administered on day one of each 21-day cycle, accompanied by oral ceralasertib for two to 17 days, either concurrently with carboplatin, or following a two-day break period. The recommended phase II dose was established as 40 mg ceralasertib, once a day, on days one and two of each cycle. Two patients demonstrated partial responses and 18 patients demonstrated stable disease. Due to dose-limiting thrombocytopenia and neutropenia events, the study authors suggest lengthening the treatment cycle to 28 days in phase II, to provide more time for bone marrow recovery. This may necessitate switching from carboplatin to cisplatin, which is more commonly used in 28-day cycles. This study was highlighted in the October 1st issue.
Journal: Cancer Research (October 15 issue)
The tumor microenvironment (TME) plays a critical role in metastatic dissemination. Macrophages are among the most abundant components in breast cancer metastatic lesions. Research suggests that tumor-associated macrophages (TAMs) promote metastasis through various mechanisms including suppressing the antitumor immune response. In this study, the authors applied single-cell RNA-seq to analyze the macrophages isolated from the lungs of mice bearing metastasis from primary breast tumors. The gene expression profiles revealed the presence of different macrophage populations, highlighting the diversity of macrophages present within the metastatic microenvironment. The most abundant of these populations expressed genes implicated in lipid metabolism as well as remodeling of the extracellular matrix and immunosuppression, while they showed reduced phagocytosis. The authors found a dramatic expansion in the number of these cells in metastasis-bearing lungs compared to controls, identifying a previously uncharacterized population of lipid-associated macrophages in lung metastasis.
Journal: Cancer Discovery
Discovery and Features of an Alkylating Signature in Colorectal Cancer
Consumption of red meat is a known risk factor for colorectal cancer. Results from preclinical experiments have suggested that eating red meat may lead to the formation of carcinogenic compounds in the colon; however, a direct molecular link between red meat consumption and colorectal cancer in patients has been lacking. The authors of this study sequenced DNA from matched normal and colorectal tumor tissues from 900 patients with colorectal cancer, all of whom had also provided information on their diet and lifestyle during the years prior to their colorectal cancer diagnoses. DNA sequencing revealed the presence of an alkylating signature that was associated with pre-diagnosis intake of red meat, but not poultry or fish. In addition, the researchers identified the genes KRAS and PIK3CA as potential targets of alkylation-induced mutation, and they found that colorectal tumors harboring KRAS or PIK3CA driver mutations had greater enrichment of the alkylating signature. They also observed that high levels of alkylating damage in colorectal tumors were associated with a greater risk of colorectal cancer-specific death in patients. “These findings suggest that red meat consumption may cause alkylating damage that leads to cancer-causing mutations in KRAS and PIK3CA, thereby promoting colorectal cancer development,” said the study’s senior author, Marios Giannakis, MD, PhD, in an AACR news release. This article is highlighted in the October issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Early-onset colorectal cancer (EOCRC), which is diagnosed in patients younger than 50 years, is becoming more frequent. The authors of this study evaluated cancer characteristics and outcomes in 2,135 patients with EOCRC who had pre-existing hereditary syndromes (146 patients), pre-existing inflammatory bowel disease (87 patients), or sporadic colorectal cancer (1,902 patients). They found pre-existing conditions (either hereditary syndromes or inflammatory bowel disease) were more common among the youngest patients (ages 18-29 years) compared with older patients. Sporadic EOCRC was more common among patients that were older, female, and had higher BMI. Pre-existing inflammatory bowel disease was associated with higher rates of metastasis, poor differentiation, adverse histology, lymphovascular and perineural invasion, and lower rates of survival compared with patients with sporadic EOCRC. In contrast, patients with hereditary syndromes had higher survival rates than those with sporadic EOCRC. Individual hereditary syndromes differed in their association with survival. The authors conclude that tumor characteristics and patient outcomes may differ between patients with pre-existing hereditary conditions and those with sporadic EOCRC. This article is highlighted in the October issue. A related commentary can be found here.
Journal: Clinical Cancer Research (October 15 issue)
Mutations in the BRCA1/2 genes found in breast cancer cause defects in an important DNA repair mechanism that render cancer cells sensitive to PARP inhibitor drugs, which block an alternative DNA repair pathway. Niraparib is a potent oral PARP inhibitor that was approved for treatment of ovarian and prostate cancers with BRCA1/2 mutations. This study presents the final results of the BRAVO phase III clinical trial that assessed the potential of niraparib in breast cancer by comparing the efficacy and safety of niraparib monotherapy with standard-of-care chemotherapy regimens in women with advanced, BRCA1/2-mutated, HER2-negative breast cancer. After the interim analysis, the trial stopped recruiting new patients because it failed to demonstrate a benefit of niraparib over chemotherapy (median duration of progression-free survival was 4.0 months with niraparib and 4.6 months with chemotherapy). In addition, there was substantial discordance between local and central review, which prevented robust comparison between arms. However, at the final analysis, the investigators observed an objective response rate of 35 percent in the niraparib arm, which confirmed that the drug has an activity in this patient population. According to the authors, this study suggests that niraparib should be further explored in breast cancer patients with BRCA mutations. This article is highlighted in the October 15 issue.
Journal: Molecular Cancer Research
Bone is a primary site of metastasis for breast cancer cells, which can travel through the bloodstream, settle in the bone, and form metastatic lesions. Therefore, preventing the growth of bone-tropic tumor cells is a topic of interest. The radiopharmaceutical radium-223 dichloride (223RaCl2) preferentially localizes to bone and emits alpha particles, which can damage cancer cells but spare bone marrow due to their short range. Recent data, however, have shown that bystander effects of 223RaCl2—signals emitted by irradiated cells that can affect non-irradiated cells—can damage both tumor cells and bone cells outside the intended range. In this study, the researchers injected breast cancer cells into irradiated mouse tibias, then harvested the tibias to evaluate DNA damage and apoptosis in both irradiated and bystander tumor cells and osteocytes. Bystander breast cancer cells from two cell lines (MDA-MB-231 and MCF7) showed DNA damage at high doses of 223RaCl2 (600 kBq/kg), but only MDA-MB-231 bystander cells and osteocytes showed DNA damage at low doses of 223RaCl2 (50 kBq/kg). Similarly, apoptosis was only evident in osteocytes and bystander MDA-MB-231 cells. The researchers speculate that intrinsic differences in the breast cancer cells, such as estrogen receptor positivity and p53 mutation status, may explain the differential sensitivity and warrant further investigation. Overall, these data demonstrate the powerful effects of 223RaCl2 on both tumor cells and the bone microenvironment. This study was highlighted in the October issue.
Journal: Cancer Research (October 1 issue)
The Evolving Genomic Landscape of Esophageal Squamous Cell Carcinoma Under Chemoradiotherapy
Esophageal squamous cell carcinoma (ESCC), a type of esophageal cancer, is frequently treated with chemoradiotherapy (CRT), a combination of chemotherapy and radiation. However, about 30 percent of patients experience a recurrence following CRT, indicating resistance development, the mechanisms of which are unknown. In this study, the researchers performed whole exome sequencing on matched pre-treatment and post-treatment ESCC samples from 33 patients, to identify driver mutations that arise during CRT. They found that most driver mutations present in recurrent tumors were also present prior to treatment, suggesting that CRT is less likely to promote the development of new mutations and more likely to select for existing ones. Mutation signatures of recurrent tumors were marked by a higher frequency of deletions, base substitutions characteristic of platinum-based chemotherapy, and increased copy number of the oncogenic Myc gene. The researchers found that ESCC cell lines overexpressing Myc were more resistant to radiotherapy, and that ESCC patients with Myc copy number amplifications before CRT had significantly shorter progression-free survival and overall survival following CRT treatment. These data help clarify the clonal evolution of ESCC tumors undergoing treatment and identify Myc amplification as a possible biomarker of CRT response.
Journal: Cancer Immunology Research
Colorectal cancers with high microsatellite instability (MSI-H) or defective mismatch repair (dMMR) are more likely to respond to immune checkpoint inhibition. However, MSI-H/dMMR status is not sufficient to predict response. Prior studies have suggested that the presence of certain tumor infiltrating immune-cell subsets may be associated with response to immunotherapy, but these studies have been largely restricted to melanoma and lung cancer. Furthermore, prior studies examining the role of residency and exhaustion programs have largely focused on those of CD8+ T cells, with little research on those of CD4+ T-cells or natural killer cells. Here, researchers analyzed RNA sequencing and transcriptomics data to identify signatures of residency and exhaustion in colorectal cancer-infiltrating CD8+ T cells, CD4+ T cells, and natural killer cells, as well as to evaluate the association of these signatures with patient outcomes. They found that patients whose tumors had high exhaustion and low residency programs in natural killer cells, or patients whose tumors had high exhaustion programs in CD8+ T cells and natural killer cells with low TGFβ signaling, had improved survival outcomes. The authors propose that these signatures may help predict survival outcome and/or response to immunotherapy in patients with colorectal cancer. This article is featured on the cover of the October issue.
Journal: Cancer Prevention Research
Patients with pancreatic cancer face a relatively poor prognosis, and prevention strategies for individuals at high risk of the disease are limited. Current screening measures, such as MRI and endoscopic ultrasound, cannot identify pancreatic intraepithelial neoplasia (PanIN), the most common precursor lesion to pancreatic cancer. The authors of this study previously developed a vaccine against the stomach protein gastrin, which is ectopically expressed in pancreatic lesions and stimulates cancer development. They showed in a previous study that their vaccine, called polyclonal antibody stimulator (PAS), could shrink pancreatic tumors, increase immune cell infiltration, and decrease metastases in mice. In this study, the researchers wanted to evaluate whether PAS could also prevent the development of pancreatic cancer if administered to mice with PanIN lesions. Mice genetically engineered to develop PanIN were initially vaccinated at three months of age and given boosters after one week, three weeks, and every subsequent four weeks until the mice were eight months old. The researchers found that vaccinated mice developed significantly fewer high-grade PanIN lesions and a lower incidence of pancreatic cancer, compared with unvaccinated mice. They also found that vaccinated mice had less pancreatic fibrosis, which prevents immune involvement; fewer tumorigenic M2 macrophages around pancreatic lesions; and increased infiltration of antitumor CD8+ T cells. The researchers suggest that PAS could potentially be administered to patients at an increased risk of pancreatic cancer. This study was featured on the cover of the October issue.



