Bringing Awareness to Breast Cancer in October
Breast cancer can be a scary diagnosis. Even though mortality rates have declined in recent years in the United States, it is estimated that over 40,000 women and roughly 500 men will die from breast cancer in 2019. Despite progress in developing new treatment modalities for patients with this disease, there is still substantial work to be done in the field.
October is Breast Cancer Awareness Month. In this post, we’ve compiled the latest approvals from the U.S. Food and Drug Administration (FDA) for the treatment of breast cancer, a selection of recent breast cancer research articles, and AACR initiatives in this area.
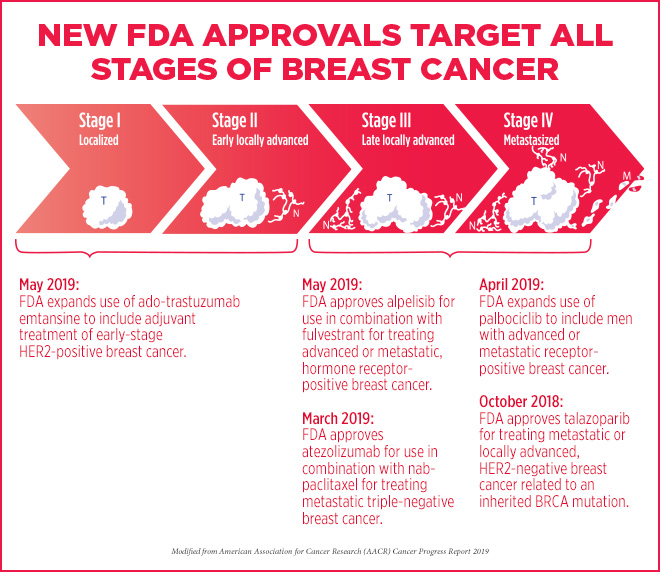
Roundup of recent FDA-approved treatments for breast cancer
Between October 2018, and May 2019, the FDA approved several treatment approaches for various types of breast cancer, as highlighted in the AACR Cancer Progress Report 2019:
- In October 2018, the FDA approved a new therapeutic, talazoparib (Talzenna), for patients harboring germline, cancer-associated BRCA1 or BRCA2 mutations with locally advanced or metastatic HER2-negative breast cancer. Talazoparib targets ADP-ribose polymerase (PARP) proteins, key players in DNA damage repair; inhibition of these repair mechanisms can lead to cancer cell death in those with mutant BRCA.
- In March 2019, the first immunotherapy regimen for breast cancer was approved. The combination of the PD-L1 inhibitor atezolizumab (Tecentriq) with the chemotherapeutic nab-paclitaxel (Abraxane) was granted accelerated approval by the FDA for adult patients with unresectable, locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1. Eva Joseph, a breast cancer survivor who was treated with this combinatorial therapy, shared her story in the AACR Cancer Progress Report.
- In April 2019, the FDA expanded the use of the cyclin-dependent kinase (CDK) inhibitor palbociclib (Ibrance), in conjunction with endocrine therapies, to include men with advanced or metastatic hormone receptor (HR)-positive, HER2-negative breast cancer. Palbociclib inhibits CDK4/6 to disrupt the cell cycle and kill rapidly dividing cancer cells. Kirby Lewis, who is currently taking palbociclib and the estrogen receptor antagonist fulvestrant for his metastatic breast cancer, talks about his experience here.
- In early May 2019, the FDA approved the expansion of the antibody-drug conjugate ado-trastuzumab emtansine (T-DM1; Kadcyla) as an adjuvant treatment for patients with early-stage HER2-positive breast cancer with residual invasive disease following neoadjuvant taxane and trastuzumab-based treatment. The FDA previously approved T-DM1 as a treatment for patients with metastatic HER2-positive breast cancer that had progressed following a prior regimen including trastuzumab and a taxane.
- Later in May, the first PI3K inhibitor for the treatment of breast cancer was approved by the FDA – alpelisib (Piqray) in combination with fulvestrant for men or postmenopausal women with advanced or metastatic HR-positive, HER2-negative, PIK3CA-mutated breast cancer that has progressed during or after endocrine therapy.
Sex-based breast cancer survival
While the vast majority of breast cancer cases occur in women, men are still susceptible to the disease. Risk factors in men include a family history of breast cancer, the presence of other conditions with high levels of estrogen (such as cirrhosis or Klinefelter syndrome), or prior radiation treatments focused to the chest.
A study recently published in JAMA Oncology found that male breast cancer patients had significantly higher rates of mortality than female breast cancer patients.
The researchers evaluated overall survival and three- and five-year mortality rates of over 16,000 male breast cancer patients and nearly 2 million female breast cancer patients. Men had a worse prognosis in all categories compared with women– they had worse overall survival (45.8 percent versus 60.4 percent), worse three-year survival (86.4 percent versus 91.7 percent), and worse five-year survival rates (77.6 percent versus 86.4 percent).
Even after the researchers adjusted for clinical characteristics, treatment, age, race/ethnicity, and access to care, men still had higher mortality compared with women. The authors posit that this disparity could be caused by factors such as biological attributes, treatment compliance, and lifestyle factors, and that additional research is needed to mitigate these survival differences.
The FDA recently released a draft guidance for the inclusion of males in clinical trials evaluating breast cancer drugs.
Fertility drugs and breast cancer risk
As women continue to postpone parenthood in the U.S., the use of fertility treatments has continued to rise. Because fertility drugs can increase estrogen levels, it has been hypothesized that such treatments might increase the risk of breast cancer, but research in this area has yielded inconclusive findings.
A study recently published in Cancer Epidemiology, Biomarkers & Prevention that evaluated the association between the use of fertility drugs and the incidence of breast cancer in over 1 million women found that the use of such drugs in infertile women did not increase the risk of breast cancer.
The researchers investigated the use of the fertility drugs clomiphene citrate, gonadotropins (including follicle-stimulating hormones and human menopausal gonadotropins), human chorionic gonadotropins (hCG), gonadotropin-releasing hormone analogues, and progesterone among Danish women aged 20-44 between January 1, 1995, and December 31, 2011.
After two decades of follow-up, compared with fertile women, infertile women who were treated with any type of fertility drugs did not have an increased risk for breast cancer. Further, there were no associations observed between the use of specific types of fertility drugs and incidence of breast cancer.
Accelerating the progress against breast cancer
The American Association for Cancer Research (AACR) has a variety of initiatives to further breast cancer research, including targeted meetings, AACR grants, and AACR awards.
Each year, the AACR partners with the Cancer Therapy & Research Center at University of Texas Health Science Center San Antonio and the Baylor College of Medicine to host the San Antonio Breast Cancer Symposium, a five-day conference focused solely on cutting-edge research in the field of breast cancer. This year, the symposium will be held Dec. 10-14. In addition to showcasing the latest breast cancer clinical trial data, the conference will feature discussions around breast cancer cell fate and plasticity, immunosuppression and the microenvironment, and the implementation of genetic testing, among other topics. The early registration deadline for the meeting is Thursday, October 31.
Interested in learning more about research in this area? The AACR journal Molecular Cancer Therapeutics has compiled a collection of seven articles highlighting new therapeutic approaches in breast cancer, which was published in this month’s issue. Read the commentary about this collection of impactful articles here.