From the Journals: Editors’ Picks for November
Each month, the editors from the portfolio of scientific journals published by the American Association for Cancer Research (AACR) highlight one “must read” article from every journal issue, which we summarize here. This month’s edition is stuffed with studies relating to recent clinical trials, a preclinical investigation of NSAIDs for the inhibition of colon tumor progression, and many more. As always, articles featured here are freely available for a limited time.
Journal: Clinical Cancer Research (November 1 issue)
The VEGFR2 inhibitor vandetanib has shown some success in tumors that have deficient expression of succinate dehydrogenase (SDH); whether this agent is efficacious in patients with SDH-deficient gastrointestinal stromal tumors (dSDH GIST) has yet to be studied. This study reports on results from a small phase II clinical trial that evaluated vandetanib in nine patients (with ages ranging from 11-52) with metastatic dSDH GIST; eight of these patients had received prior treatment with a tyrosine kinase inhibitor. While three of the initial five adult patients who received 300mg of vandetanib daily developed treatment-modifying toxicities, two pediatric patients who received 150mg/m2 of vandetanib daily did not experience such toxicities. No partial or complete responses were observed, but two patients experienced prolonged stable disease. The authors conclude that vandetanib is not active in dSDH GIST, highlighting the need for novel therapeutic strategies for this group of patients. This article was highlighted in this issue.
Journal: Cancer Research (November 1 issue)
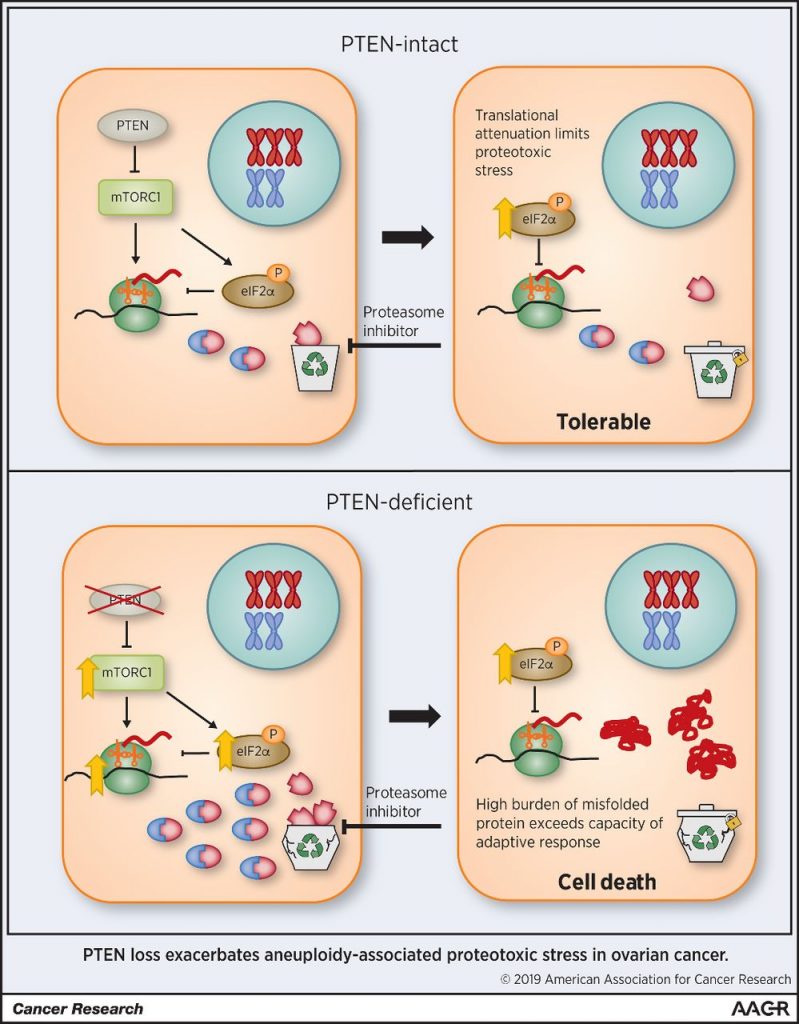
High-grade serous ovarian carcinoma is characterized by a high level of chromosomal instability, making it vulnerable to proteasome inhibition. However, in a phase II clinical trial, only a small minority of patients with ovarian cancer showed clinical benefit when treated with the proteasome inhibitor bortezomib. In this study, the authors showed that chromosome missegregation in immortalized tubal epithelial cells was associated with enhanced susceptibility to proteasome inhibition. Although adherent ovarian cancer cells were vulnerable to proteasome inhibition, spheroid models of ovarian cancer were resistant. Resistance to proteasome inhibition was due to suppression of mTORC1, which resulted in downregulation of protein synthesis. Depletion of PTEN led to constitutive activation of mTORC1, enhanced proteotoxic stress, and resensitized cells to proteasome inhibition. The authors hypothesize that attenuation of protein synthesis in tumor spheroids may explain the overall poor response to bortezomib in clinical trials. The authors propose that patients with PTEN-deficient tumors may be amenable to treatment with proteasome inhibitors or other therapies that disrupt protein homeostasis. This article was featured on the cover of this issue.
Journal: Cancer Research (November 15 issue)
Establishing the Impact of Vascular Damage on Tumor Response to High-Dose Radiation Therapy
While stereotactic body radiation therapy (SBRT) is currently utilized as a treatment for a wide variety of cancers, the underlying mechanism by which this treatment eradicates tumor cells more effectively than conventional radiotherapy – either indirectly, by damaging the tumor vasculature, or directly, due to the increased radiation dose – remains controversial. To better understand this issue, the authors used genetically engineered mouse models of sarcoma and lung cancer to selectively delete the ATM gene, which hypersensitizes cells to radiation, in either endothelial cells or primary tumor cells. They found that SBRT exposure had a bigger impact on tumor cells than endothelial cells in the local tumor control of both cancer models. These results indicate that the major therapeutic benefit of SBRT is derived from the direct killing of tumor cells. This article was featured on the cover of this issue.
Journal: Molecular Cancer Therapeutics
The RNA helicase p68, when phosphorylated, is associated with tumorigenic transformation, cancer progression, invasion, and metastasis, and is aberrantly expressed in triple negative breast cancer (TNBC) cells. This study evaluated the first-in-class phosphorylated-p68 inhibitor RX-5902 in preclinical models of TNBC. The researchers found that RX-5902 was active in both TNBC cell lines and in TNBC patient-derived xenograft (PDX) models, and gene set enrichment analysis revealed that treatment with RX-5902 resulted in a decrease in the expression of β-catenin and MCL-1, which may mediate apoptosis and cell death. The authors note that a phase II clinical trial evaluating RX-5902 in patients with TNBC is currently enrolling. This article was highlighted in this issue.
Journal: Cancer Discovery
Infiltrating Myeloid Cells Drive Osteosarcoma Progression via GRM4 Regulation of IL23
A genome-wide association study previously linked a locus within the glutamate metabotropic receptor 4 (GRM4) gene to osteosarcoma susceptibility, but the biological role of GRM4 in osteosarcoma remains unclear. In this study, the authors demonstrated that Grm4-/- mice had accelerated tumor development in response to radiation compared to wild-type mice, suggesting that GRM4 has a tumor-suppressive effect. Grm4-/- mice exhibited increased expression of the IL23 cytokine. The authors found that Il23-/- mice were resistant to osteosarcoma development, and higher IL23 expression was associated with worse survival outcomes in human patients. Agonists of GRM4 or a neutralizing antibody to IL23 suppressed osteosarcoma development in mice. Together, the results implicate GRM4 and IL23 as potential therapeutic targets for the treatment of osteosarcoma. This article was highlighted in this issue. A related commentary can be found here.
Journal: Cancer Prevention Research
A growing body of research indicates that non-steroidal anti-inflammatory drugs (NSAIDs) are beneficial for the prevention of colorectal cancer. However, long-term use of NSAIDs is associated with gastrointestinal toxicities and other adverse effects. In this study, the authors used an azoxymethane-rat colon cancer model to assess the efficacy and toxicity of different doses of aspirin or naproxen administered through diet. Rats received either aspirin or naproxen at one of the different test doses, which were administered for 48 weeks continuously, one week on/one week off, or three weeks on/three weeks off. No overt toxicities were observed. The different treatment regimens of both agents resulted in dose-dependent inhibition of tumor progression. Modulation in tumor biomarkers of apoptosis, proliferation, and proinflammatory mediators were significantly correlated with tumor inhibition. The authors conclude that intermittent dosing with aspirin or naproxen are effective in inhibiting the progression of colon tumors without gastrointestinal toxicities. This article was featured on the cover of this issue.
Journal: Molecular Cancer Research
Epidermal growth factor receptor (EGFR) is overexpressed in many cancer types and has been extensively explored as a molecular target, yet few studies have attempted to understand how this key protein is transcriptionally regulated. In this study, the researchers identified two critical constituent enhancers in the first intron of the EGFR gene in models of glioblastoma (GBM) and head and neck squamous cell carcinoma (HNSCC). Genetic deletion or targeted repression of these enhancers significantly reduced the transcription of EGFR. Additionally, the authors found that the proteins AP-1 and BET modulate these enhancers in GBM and HNSCC, and disruption of BET/AP-1 binding downregulates EGFR protein and mRNA transcript levels. The authors conclude that perturbation of BET and AP-1 is a potential therapeutic strategy to target essential EGFR enhancers in EGFR-positive malignancies. This article was highlighted in this issue.
Journal: Clinical Cancer Research (November 15 issue)
While some patients with estrogen receptor (ER)-positive breast cancer benefit from a therapeutic regimen that combines endocrine therapy with PI3K-mTOR inhibition, this treatment strategy is limited by its substantial toxicity. This study reports on results from a phase Ib clinical trial that evaluated the combination of tamoxifen with taselisib, an isoform-selective inhibitor of PI3K, in 30 patients with ER-positive metastatic breast cancer that had progressed during prior endocrine therapy. Among the 25 evaluable patients, the objective response rate was 24 percent, and 12 out of the 30 patients (40 percent) had disease control for at least six months. Adverse events were consistent with this class of drugs and included diarrhea (43 percent of patients), mucositis (33 percent of patients), and hyperglycemia (27 percent of patients); no dose-limiting toxicities were observed. The randomized, phase II portion of this trial, which is evaluating tamoxifen plus taselisib or placebo, is currently recruiting. This article was highlighted in this issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Family history is an important factor in assessing risk for breast cancer. Information regarding family history usually relies on self-reporting from the patient. Few studies have examined the accuracy of self-reporting in racial and ethnic minorities. Here, the authors assessed the accuracy of self-reports of family history of cancer by women with breast cancer from the Northern California Breast Cancer Family Registry. Self-reported information was compared to personal cancer history reports provided by the patients’ female first-degree relatives and to records from the California Cancer Registry. The authors found that patients reported breast cancer in their first-degree relatives with high accuracy but were less accurate when reporting other cancers. Accuracy did not vary significantly by race, ethnicity, age, or education. Although not statistically significant, family history of cancer reported by Hispanic white patients was marginally less accurate than that reported by non-Hispanic white patients. When using the California Cancer Registry as a reference, the authors found that the accuracy of self-reported family history of breast cancer was significantly lower for patients with low English language proficiency. The authors conclude that self-reported family history of breast cancer in first-degree relatives was highly accurate for non-Hispanic whites, African Americans, and Asian Americans, but lower for Hispanic whites and those with limited English language proficiency. The authors suggest that self-reported family history can be used when other methods are not available. This article was highlighted in this issue.
Journal: Cancer Immunology Research
The Atypical Receptor CCRL2 Is Essential for Lung Cancer Immune Surveillance
CCRL2 is a nonsignaling seven-transmembrane domain receptor that binds a chemotactic protein called chimerin. The binding of chimerin to CCRL2 promotes chemotaxis of leukocytes, such as macrophages and natural killer cells, and controls the inflammatory response. In this study, the authors examined the role of CCRL2 in regulating inflammation in the context of lung cancer. The authors found that deletion of the Ccrl2 gene in mice promoted progression of Kras-mutated and p53-mutated lung tumors. This effect was associated with impaired recruitment of several leukocyte populations. The authors determined that CCRL2 expression in nonhematopoietic cells was responsible for the impact on tumor progression. Consistent with the results in mice, elevated CCRL2 expression in biopsies from human lung adenocarcinoma positively correlated with clinical outcome. Together, the results suggest that CCRL2 contributes to an anti-lung tumor immune response. This article was featured on the cover of this issue.