Immunotherapy-Targeted Therapy Combination Treatment for Endometrial Cancer
The FDA approval of the combination therapy further expands the number of cancers that can be treated with pembrolizumab.
The U.S. Food and Drug Administration (FDA) recently approved an immunotherapy-molecularly targeted combination to treat patients with advanced endometrial cancer, also called uterine cancer.
More than 60,000 women are diagnosed with endometrial cancer and some 12,000 die of the disease each year, according to the National Cancer Institute. The five-year survival rate for endometrial cancer is 81.2 percent, but just 18 percent for advanced disease that has metastasized to distant sites.
The FDA approved the immune checkpoint inhibitor pembrolizumab (Keytruda) in combination with the molecularly targeted therapeutic lenvatinib (Lenvima) for women with advanced disease who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The action further expands the number of cancers for which pembrolizumab is now a treatment option. The decision was based on results from a phase Ib/II clinical trial that included 94 patients with advanced endometrial cancer that was neither microsatellite instability-high nor mismatch repair-deficient. According to the FDA statement, 10 of these patients had a complete response (10.6 percent) and 26 had a partial response (27.7 percent). At the time of the approval, the median duration of the response had not been reached.
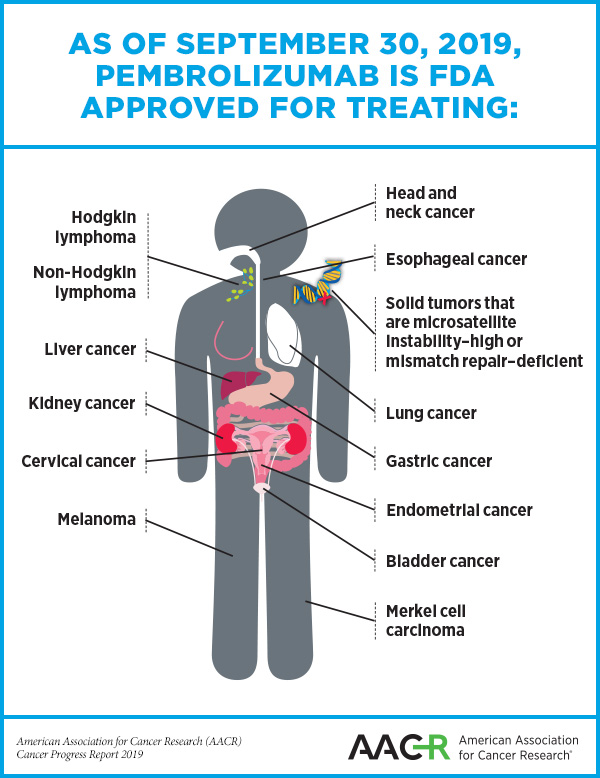
The Australian and Canadian regulatory agencies approved pembrolizumab for endometrial cancer at the same time as the FDA as a result of a new FDA initiative called Project Orbis, designed to increase collaboration among regulatory agencies in different countries. This is the first time that the FDA has collaborated in this way with international partners, but the agency hopes that future collaboration through this initiative will help cancer patients around the world receive earlier access to groundbreaking new treatments.
Learn more about the September 17, 2019, approval on Cancer Research Catalyst, the official blog of the American Association for Cancer Research.
