Secrets of the Sea to Treat Lung Cancer
The FDA approved an analog of a marine compound to treat certain patients with small cell lung cancer.
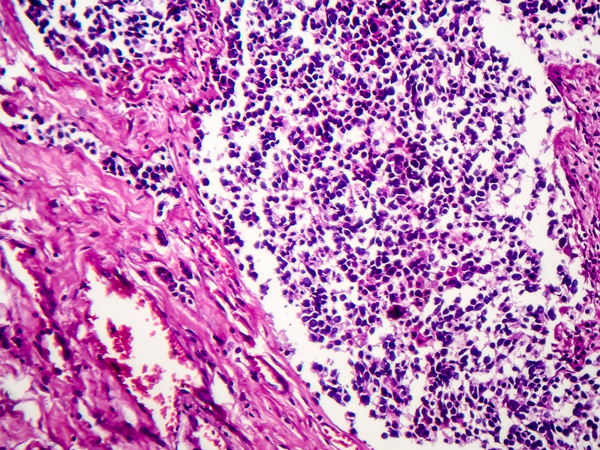
The U.S. Food and Drug Administration granted accelerated approval to lurbinectedin (Zepzelca) for adult patients with metastatic small cell lung cancer (SCLC) that has continued to progress after treatment with a platinum-based chemotherapy agent. Lurbinectedin is an analog of a compound isolated from sea squirts, which are potato-shaped organisms that reside on pier pilings, ship hulls, rocks, large seashells, or on the backs of large crabs. This anticancer agent inhibits cellular transcription processes that many tumors depend on for growth.
The approval was based on efficacy demonstrated in a multicenter open-label, multi-cohort study. The main outcome measure was overall response rates among 105 patients, which was 30 percent, with a median response duration of 5.1 months.
Small cell lung cancer is one of the two main types of lung cancer. According to estimates by the National Cancer Institute (NCI), about 228,820 patients will be diagnosed with lung and bronchus cancer and 135,720 patients will die of the disease in 2020, making lung cancer the most common cause of cancer death among both men and women. Some 10 percent to 15 percent of lung cancers are estimated to be the small cell kind, in which cancer cells form in the tissues of the lung.
Lurbinectedin was granted accelerated approval by the FDA on June 15, 2020. Accelerated approval means continued approval may be contingent upon a confirmatory trial.
