Taking Aim at a Rare Type of Thyroid Cancer
The FDA has expanded the approval of a molecularly targeted therapeutic to treat certain pediatric and adult patients with medullary thyroid cancer.
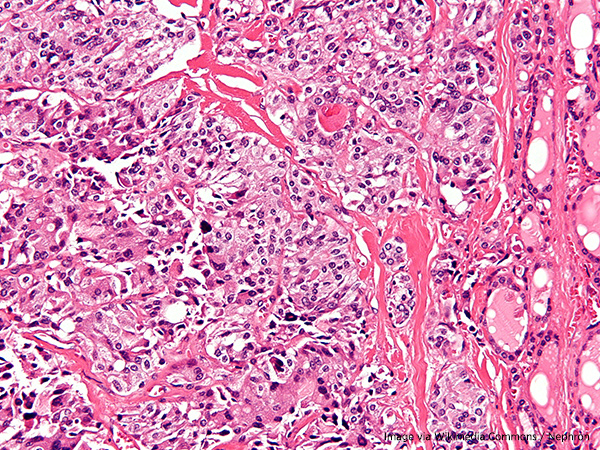
The U.S. Food and Drug Administration has approved pralsetinib (Gavreto) to treat certain adult and pediatric patients age 12 and over who have advanced or metastatic medullary thyroid cancer.
The approval was for patients whose cancers have alterations in the RET gene and who require systemic therapy or patients with RET fusion-positive disease who do not respond or are no longer responding to treatment with radioactive iodine, which is often the primary first-line therapy.
Pralsetinib is a molecularly targeted therapy designed to inhibit the function of abnormal proteins created by a change in the RET gene or by a chemical process called fusion that occurs in tumors that are known as RET fusion-positive. The abnormal proteins produced by RET alterations help cancer cells grow.
Data demonstrating the efficacy of pralsetinib stems from a multicenter, open-label, multi-cohort clinical trial involving patients with tumors that tested positive for RET alterations. The first cohort involved 55 patients who received previous treatment with either cabozantinib (cabometyx) or vandetanib (Caprelsa) —two other targeted therapeutics that take aim at RET-altered thyroid cancers. The overall response rate among this group was 60 percent, with 79 percent of the responders having response times lasting six months or longer. The second group involved 29 patients who did not receive treatment with the other targeted agents. Among this group, the overall response rate was 66 percent, with 84 percent of the responders experiencing response durations lasting six months or more.
Additional data was obtained from nine patients with RET fusion-positive thyroid cancer whose cancer did not respond to treatment with radioactive iodine. The overall response rate among these nine patients was 89 percent, with all of the respondents in this group experiencing responses lasting six months or longer.
Medullary thyroid cancer is the rarest form of thyroid cancer, comprising 3 percent to 4 percent of all thyroid cancer diagnoses in the United States each year, according to the National Cancer Institute. This type of thyroid cancer occurs in the center of the thyroid, which is a gland in the front of the neck that is responsible for sending out hormones throughout the body. Roughly 1,000 people in the U.S. are diagnosed with medullary thyroid cancer each year.
The FDA approval was rendered on December 1, 2020.
