Targeting a Protein to Treat Aggressive Blood Cancer
The FDA has approved a targeted therapy drug to treat certain adult patients with diffuse large B-cell lymphoma.
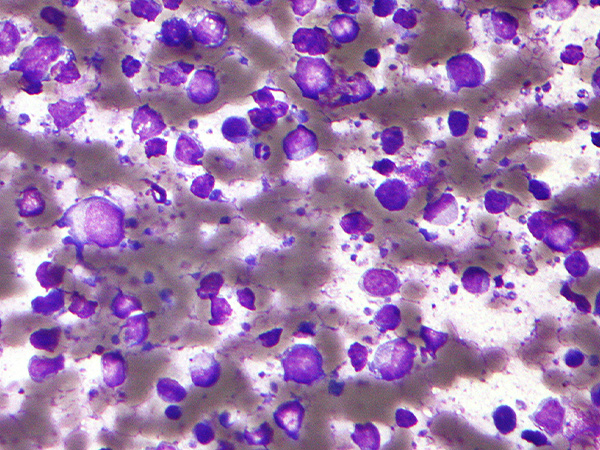
The U.S. Food and Drug Administration (FDA) has granted accelerated approval of the molecular targeted therapeutic selinexor (Xpovio) to treat adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), including the kind that arises from follicular lymphoma, which is a more slow-growing form of the disease. The molecularly targeted therapeutic was approved for use in adult patients who have received at least two previous systemic therapies and whose cancer either came back or failed to respond to those treatments.
Selinexor inhibits the function of a protein known as XPO1, which helps shuttle many key cellular regulators out of the nucleus of cells and is overexpressed in many cancers. Research studies have shown that inhibition of XPO1 causes tumor suppressor proteins to remain in the nucleus where they can act to disrupt the growth of cancer cells.
This approval was based on an overall response rate of 29 percent among 134 participants in a multicenter, single-arm, open-label trial. Out of the 39 patients who had either a complete or partial response to the drug, 38 percent had a response duration of at least six months and 15 percent had a response duration of at least 12 months.
Diffuse large B-cell lymphoma is an aggressive cancer and one of the most common forms of non-Hodgkin lymphoma, which is cancer that affects white blood cells in the body’s immune system. Diffuse large B-cell lymphoma develops in B cells and often starts with a fast-growing mass in a lymph node under the arm or in the neck or chest area. This type of cancer can also develop in other parts of the body, such as the intestines, bones, or the brain or spinal cord.
According to federal statistics, there will be more than 77,000 new cases of non-Hodgkin lymphoma diagnosed in the U.S. in 2020. Diffuse large B-cell lymphoma accounts for roughly 25 to 30 percent of B-cell non-Hodgkin lymphoma.
Selinexor was granted accelerated approval on June 22, 2020. Accelerated approval means continued approval may be contingent upon a confirmatory trial.
