A New Cancer Inhibitor and Diagnostic Test for Early, High-risk Breast Cancer
THE FDA APPROVED A NEW TYPE OF CANCER INHIBITOR FOR CERTAIN PATIENTS WITH A HIGH RISK OF RECURRENCE
The U.S. Food and Drug Administration (FDA) approved abemaciclib (Verzenio) with endocrine therapy for certain patients with early breast cancer that has a high risk of coming back after surgery.
Abemaciclib is a type of kinase inhibitor that may prevent the growth of cancer cells by blocking certain proteins known as cyclin-dependent kinases (CDKs). It is the first CDK4/6 inhibitor approved for adjuvant treatment of breast cancer.
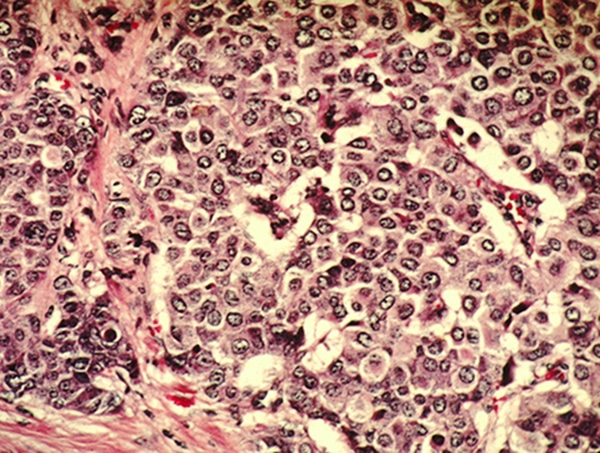
The indication is for patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer with a high risk of recurrence and a score of 20 percent or more on an FDA-approved companion diagnostic lab test. The test, called the Ki-67 IHC MIB-1 pharm Dx, is used to analyze a biopsy sample. The score measures the level of cancer cells expressing the biomarker Ki-67, which is associated with rapid growth.
The efficacy of abemaciclib was evaluated in a randomized, open-label, multicenter clinical trial involving women and men with HR-positive, HER2-negative, node-positive early breast cancer that had been removed surgically and had a high risk of coming back according to clinical features and evidence from biopsy samples. Patients received either oral abemaciclib plus their physician’s choice of standard endocrine therapy (tamoxifen or an aromatase inhibitor) or standard oral endocrine therapy alone for two years.
Among 2,003 patients with a high risk of recurrence and a Ki-67 score of 20 percent or more, the risk of invasive disease was 37 percent lower in those who received abemaciclib plus standard endocrine therapy compared to patients who received standard endocrine therapy alone.
It was estimated that 281,550 women would be diagnosed with breast cancer in 2021 in the United States. Men may also develop breast cancer and represent slightly less than 1 percent of breast cancer cases.
The FDA rendered the decision on October 12, 2021.