Editors’ Picks, July 2025: U.S. Cancer Mortality Hotspots, Chemotherapy-induced Hearing Loss, and More
Summer may be in full swing, but this month’s Editors’ Picks from the 10 peer-reviewed journals of the American Association for Cancer Research (AACR) summoned a different kind of heat map: one that pinpointed pockets across the United States where cancer mortality rates have flared up. One study dove into the ear, exploring the mechanisms through which chemotherapy can disrupt inner ear cells and promote hearing loss, while others highlighted new immunotherapy approaches, including a tumor‑derived vaccine platform and an antibody‑drug conjugate built to disarm the immunosuppressive grip of regulatory T cells. Rounding out this edition, a new special series in Cancer Research showcased a machine learning method to identify patients with breast and ovarian cancer who might benefit from PARP inhibition.
Follow the links for the full text of each article, which are freely available for a limited time.
Journal: Blood Cancer Discovery
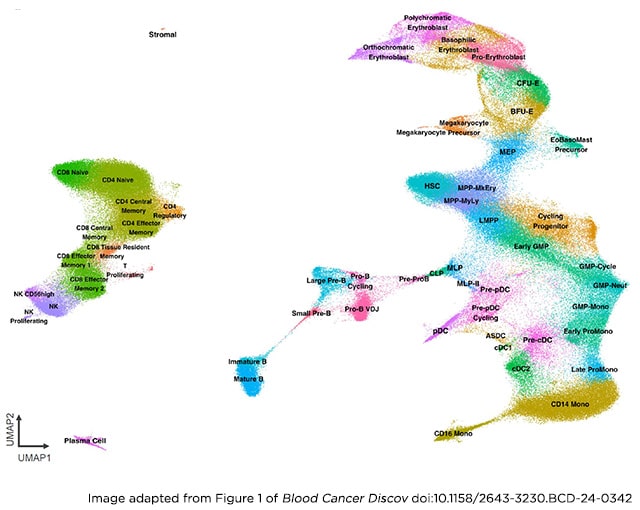
Therapeutic targeting of acute myeloid leukemia (AML) is hampered by intra- and inter-tumoral cell state heterogeneity. To develop a more precise understanding of AML cell states, we constructed a reference atlas of human hematopoiesis from 263,159 single-cell transcriptomes spanning 55 cellular states. Using this atlas, we mapped more than 1.2 million cells spanning 318 leukemia samples, revealing 12 recurrent patterns of aberrant differentiation in AML. Notably, this uncovered unexpected AML cell states resembling lymphoid and erythroid progenitors that were prognostic within the clinically heterogeneous context of normal karyotype AML, independent of genomic classifications. Systematic mapping of genotype-to-phenotype associations revealed specific differentiation landscapes associated with more than 45 genetic drivers. Importantly, distinct cellular hierarchies can arise from samples sharing the same genetic driver, potentially reflecting distinct cellular origins for disease-sustaining leukemia stem cells. Thus, precise mapping of malignant cell states provides insights into leukemogenesis and refines disease classification in acute leukemia.
Significance: We present a single-cell reference atlas of human hematopoiesis and a computational tool for rapid mapping and classification of healthy and leukemic cells. Applied to AML, this has enabled single-cell analysis at the scale of hundreds of patient samples, revealing the full breadth of derailment of differentiation in AML.
This study was simultaneously presented at the AACR Annual Meeting 2025, highlighted in a press release, and covered on the AACR blog. A related commentary was also published in the July issue.
Journal: Cancer Discovery

Cancer cell heterogeneity is a major therapeutic challenge. In this study, we identify that individual cells within cancer cell populations show significant heterogeneity in the levels of the stress-adaptive organelles, stress granules (SG), and demonstrate that SG heterogeneity is dictated by the cell cycle state. Specifically, SG formation is distinctively heightened in cells in the G2 phase because of the interplay between a nonapoptotic function of CASPASE-3 and calcium-dependent phospholipase A2 (cPLA2)–mediated production of the SG-promoting molecule, 15-deoxy-delta-prostaglandin-J2. We demonstrate that in the G1–S phase, CASPASE-3 cleaves and inactivates cPLA2, whereas in the G2 phase, CASPASE-3 activity is suppressed, resulting in enhanced cPLA2 activity and 15-deoxy-delta-prostaglandin-J2 upregulation. We show that cell cycle–dependent SG heterogeneity is a property of pancreatic ductal adenocarcinoma and targeting G2-SGs by inhibiting cPLA2 sensitizes pancreatic ductal adenocarcinoma to G2 arrest–inducing chemotherapeutics. Our findings highlight cell cycle–dependent SG formation as a fundamental property of SGs, a key aspect of cancer heterogeneity, and a target for cancer treatment.
Significance: Because of their defective G1 checkpoint mechanisms, cancer cells often activate a G2 checkpoint, which leads to resistance to DNA-damaging chemotherapeutics. We identify an intercellular heterogeneity of SGs that is driven by the cell cycle, with SG formation being highest in the G2 phase. Targeting G2-specific SG formation sensitizes pancreatic tumors to G2 arrest–inducing chemotherapeutics.
This article was featured on the cover of the July issue.
Journal: Cancer Epidemiology, Biomarkers & Prevention
Background: Identifying changes in geographic disparities of cancer mortality reveals locations where cancer prevention and control efforts should be focused/targeted. We use recent cancer surveillance data to demonstrate the geographic disparity of major cancer mortality rates in the United States and its shift compared with previous data.
Methods: This cross-sectional study used the 2018 to 2022 county-level mortality rates of colorectal, lung, breast, and prostate cancers from the Centers for Disease Control mortality data. Counties with suppressed death counts were imputed by spatial regression models. Getis–Ord Gi* statistics were used to evaluate the spatial clustering of county mortality. Identified hotspot counties were visualized and compared with literature for hotspot pattern change.
Results: A total of 3,108 U.S. mainland counties were included. Cancer mortality rates were significantly higher in 244 counties for colorectal, 456 for lung, 147 for breast, and 180 for prostate cancers. Hotspot areas were central Appalachia (colorectal and lung cancers), Lower Mississippi Delta (colorectal, breast, and prostate cancers), Midwest (colorectal and lung cancers), north Michigan/Wisconsin (lung and prostate cancers), north Florida (lung cancer), and the West (prostate cancer).
Conclusions: West central Appalachia and Lower Mississippi Delta continue to be hotspots for major cancer types, whereas previously identified eastern North Carolina/Virginia hotspots shrunk, east Oklahoma and North Florida emerged as new hotspots for lung cancer, and several hotspots emerged in the West for prostate cancer.
Impact: This study updated the analyses for geospatial disparity in major cancer mortality since 2018, illustrating recent changes in the disparity pattern and pinpointing areas that cancer prevention and control efforts should target.

Journal: Cancer Immunology Research
The optimal means to prime for effective antitumor immunity in a patient with cancer remain elusive in the current era of checkpoint blockade. Crafting a strategy to amplify the number and function of CD8+ T cells while blocking regulatory cells should increase immunotherapy efficacy. Biomaterial carriers have been demonstrated in preclinical studies to amplify the effects of immunomodulatory agents, synergistically integrate the effects of different agents, and concentrate and manipulate immune cells in vivo. Herein, we report data from a phase I trial in patients with metastatic melanoma who received the cytokine GM-CSF and the innate Toll-like receptor 9 agonist CpG oligonucleotide admixed with autologous tumor lysate onto a microporous poly-lactide-co-glycolide matrix polymer scaffold that achieves precise control over the spatial and temporal release of immunostimulatory agents in vivo. This materials system (WDVAX) served as a physical antigen-presenting structure to which dendritic cells and other immune-stimulating cells are recruited and activated. In this first clinical trial of a macroscale biomaterial–based vaccine, WDVAX treatment was found to be feasible and to induce immune activation in patients with melanoma.
This article was featured on the cover of the July issue.
Journal: Cancer Prevention Research
Historically, cancers diagnosed via the emergency department (ED) portend a poor prognosis. Recent data from the United States are sparse, and analyses of cancers detected in the years following ED visits are lacking. Thus, we analyzed data from nine rural U.S. Midwest counties included within the population-based Rochester Epidemiology Project (2015–2021). Participants without a history of cancer (N = 42,074) who did not receive ED care were matched 1:1 to ED participants on the date of ED visit, age, sex, race, ethnicity, and county of residence. Analyses were restricted to participants with records ≤2 years prior to ED or index visit and ≥30 days after. HRs and 95% confidence intervals (CI) comparing cancer incidence and deaths among ED and non-ED participants were estimated from Cox proportional hazards regression models, either unadjusted or adjusted for covariates. Cumulative cancer incidence curves accounting for competing risks of death and survival (all cause and cancer-specific) were estimated. The median follow-up was 6.3 years, with 2,719 (6.46%) cancers diagnosed among ED participants and 3,139 (7.46%) among non-ED participants. ED participants experienced lower cancer risk overall (HRAdjusted = 0.70; 95% CI, 0.66–0.74; P = 8.89 × 10−31), specifically for breast cancer, prostate cancer, melanoma, and secondary cancers. Cancer-specific mortality was higher among ED participants (HRAdjusted = 1.76; 95% CI, 1.49–2.08; P = 3.62 × 10−11). Compared with non-ED participants, ED participants experienced a lower incidence of cancer but higher overall cancer-specific mortality, suggesting that subsets of ED patients may benefit from postvisit preventive interventions.
Prevention Relevance: This cohort analysis shows that cancer incidence over 6 years was lower among participants after an ED visit than among matched non-ED participants, whereas cancer-specific mortality was higher in the ED group (HRAdjusted = 1.76; 95% CI, 1.49–2.08; P = 3.62 × 10−11), suggesting the potential benefit of preventive interventions.
Journal: Cancer Research (July 1 Issue)

Breast and ovarian cancers harboring homologous recombination deficiency (HRD) are sensitive to PARP inhibitors and platinum chemotherapy. Conventionally, detecting HRD involves screening for defects in BRCA1, BRCA2, and other relevant genes. Recent analyses have shown that HRD cancers exhibit characteristic mutational signatures due to the activities of HRD-associated mutational processes. At least three machine learning tools exist for detecting HRD based on mutational patterns. In this study, using sequencing data from 1,043 breast and 182 ovarian cancers, we trained Homologous Recombination Proficiency Profiler (HRProfiler), a machine learning method for detecting HRD using six mutational features. The performance of HRProfiler was assessed against prior approaches using additional independent datasets of 417 breast and 115 ovarian cancers, including retrospective data from a clinical trial involving patients treated with PARP inhibitors. Individual HRD-associated mutational signatures alone did not consistently detect HRD or predict clinical response across datasets. Notably, while all tools performed comparably for whole-genome–sequenced cancers, HRProfiler was the only approach that consistently identified HRD in whole-exome–sequenced breast and ovarian cancers, offering clinically relevant insights. Retrospective analyses provided strong evidence that HRProfiler could serve as a valuable tool for predicting HRD and clinical response in breast and ovarian cancers. This study provides the rationale for large-scale prospective clinical trials to validate the potential of HRProfiler as a routine predictive and/or prognostic HRD biomarker to guide clinical decision-making.
Significance: HRProfiler is a machine learning approach that reliably identifies homologous recombination deficiency in whole-exome–sequenced breast and ovarian cancers, outperforming other tools and providing clinically useful insights.
This article was featured on the cover of the July 1 issue, which also included a related commentary. The article was also included in the journal’s special series on “Driving Cancer Discoveries with Computational Research, Data Science, and Machine Learning/AI.”
Journal: Cancer Research (July 15 Issue)
Combination therapies are one potential approach to improve the outcomes of patients with relapsed/refractory (R/R) disease. However, comprehensive testing in scarce primary patient material is hampered by the many drug combination possibilities. Furthermore, inter- and intrapatient heterogeneity necessitates personalized treatment optimization approaches that effectively exploit patient-specific vulnerabilities to selectively target both the disease- and resistance-driving cell populations. In this study, we developed a systematic combinatorial design strategy that uses machine learning to prioritize the most promising drug combinations for patients with R/R acute myeloid leukemia (AML). The predictive approach leveraged single-cell transcriptomics and single-agent response profiles measured in primary patient samples to identify targeted combinations that coinhibit treatment-resistant cancer cells individually in each sample of patients with AML. Cell type compositions evolved dynamically between the diagnostic and R/R stages uniquely in each patient, hence requiring personalized drug combination strategies to target therapy-resistant cancer cells. Cell population–specific drug combination assays demonstrated how patient-specific and disease stage–tailored combination predictions led to treatments with synergy and strong potency in R/R AML cells, whereas the same combinations elicited nonsynergistic effects in the diagnostic stage and minimal coinhibitory effects on normal cells. In preliminary experiments on clinical trial samples, the approach predicted clinical outcomes of venetoclax–azacitidine combination therapy in patients with AML. Overall, the computational–experimental approach provides a rational means to identify personalized combinatorial regimens for individual patients with AML with R/R disease that target treatment-resistant leukemic cells, thereby increasing their likelihood of clinical translation.
Significance: A predictive model identifies patient-tailored combinations that coinhibit multiple drivers to selectively and synergistically target leukemia cells, which could reduce therapy resistance and enhance treatment outcomes in patients with advanced disease.
This article was also featured in the journal’s special series on computational research, data science, and machine learning/AI.

Journal: Clinical Cancer Research (July 1 Issue)
Purpose: This study investigated the effects of taxane–cisplatin combinations on pathologic complete response (pCR) rates and survival outcomes in triple-negative breast cancer (TNBC).
Patients and Methods: The HELEN-001 trial enrolled patients ages 18 to 70 years with stage II–III TNBC, randomly assigning them to receive either docetaxel (75 mg/m2) plus cisplatin (75 mg/m2; TP) or docetaxel (75 mg/m2), doxorubicin (50 mg/m2), and cyclophosphamide (500 mg/m2; TAC). Treatments were administered every 3 weeks for six cycles, with the primary endpoint being pCR (ypT0/isN0) and secondary endpoints being event-free survival (EFS), overall response rate, breast-conserving surgery rate, and toxicity.
Results: From November 2018 to June 2022, 212 Asian female patients were enrolled across six hospitals in China, with 106 patients in each group. The pCR rate was significantly higher for TP (51.9%) than for TAC (35.8%; P = 0.028). After a median follow-up of 40 months, EFS was 86.1% in the TP group and 80.0% in the TAC group (HR, 0.639; P = 0.196). In germline BRCA1/2 mutation carriers, EFS was significantly higher with TP than with TAC (100% vs. 53.8%; P = 0.008). Grade 3 or higher adverse events occurred in 54% of patients in the TP group and 48% in the TAC group.
Conclusions: The TP regimen demonstrated significantly improved pCR rates with a manageable toxicity profile, suggesting the potential benefit of taxane plus platinum regimens in patients with TNBC.
Journal: Clinical Cancer Research (July 15 Issue)
Purpose: Tifcemalimab is a recombinant humanized IgG4k monoclonal antibody targeting B- and T-lymphocyte attenuator. Co-blockade of B- and T-lymphocyte attenuator and programmed death-1 pathways improved outcomes in nonclinical models. This phase I/II trial evaluated the safety and preliminary efficacy of tifcemalimab plus toripalimab in advanced lung cancer.
Patients and Methods: Eligible patients with pathologically confirmed advanced non–small cell lung cancer (NSCLC) without sensitive EGFR variation and anaplastic lymphoma kinase fusion who failed standard treatment including one PD-1/PD-L1 inhibitor or those with refractory extensive-stage small cell lung cancer (SCLC) received tifcemalimab (200 mg) and toripalimab (240 mg) every 3 weeks intravenously until disease progression or intolerable toxicity. The Simon two-stage optimal design was used in the expansion part. The primary endpoints included safety and objective response rate (ORR) per RECIST version 1.1.
Results: Twenty-four patients with NSCLC and 43 with SCLC were enrolled (median age of all patients, 60.0 years). All patients with NSCLC and 14 (32.6%) with SCLC had received previous immunotherapy. Fifty-five (82.1%) patients experienced treatment-related adverse events, and five (7.5%) patients reported grade ≥3 immune-related adverse events. For NSCLC, the ORR was 4.3%, and disease control rate was 47.8%. The median progression-free survival and overall survival were 1.5 and 18.9 months, respectively. For SCLC, the ORR and disease control rate were 35.0% and 55.0%, respectively. The median duration of response, progression-free survival, and overall survival were 5.7, 2.8, and 12.3 months, respectively.
Conclusions: Tifcemalimab plus toripalimab showed promising antitumor activities with acceptable safety, especially in advanced refractory SCLC.
Journal: Molecular Cancer Research
Protein homeostasis is critical to the survival of multiple myeloma cells. Although this is targeted with proteasome inhibitors, mRNA translation inhibition has not entered trials. Recent work illustrates broad sensitivity of multiple myeloma cells to the translation inhibitor omacetaxine. We hypothesized that understanding how multiple myeloma becomes resistant will lead to the development of drug combinations to prevent or delay relapse. We generated omacetaxine resistance in H929 and MM1S multiple myeloma cell lines and compared them with parental lines. Resistant lines displayed decreased sensitivity to omacetaxine, with EC50 > 100 nmol/L, compared with parental sensitivity of 24 to 54 nmol/L. As omacetaxine inhibits protein synthesis, we performed both RNA sequencing and ribosome profiling to identify shared and unique regulatory strategies of resistance. Transcripts encoding translation factors and containing a terminal oligopyrimidine sequence in their 5’ untranslated region were translationally upregulated in both resistant cell lines. The mTOR pathway promotes the translation of terminal oligopyrimidine motif–containing mRNAs. Indeed, mTOR inhibition with Torin 1 restored partial sensitivity to omacetaxine in both resistant cell lines. The combination was synergistic in omacetaxine-naïve multiple myeloma cell lines, and a combination effect was observed in vivo. Primary multiple myeloma cells from patient samples were also sensitive to the combination. These results provide a rational approach for omacetaxine-based combination therapy in patients with multiple myeloma, which have historically shown better responses to multiagent regimens.
Implications: Through the use of ribosome profiling, our findings indicate mTOR inhibition as a novel combination therapy for partnering with the translation inhibitor omacetaxine in the treatment of multiple myeloma.
Journal: Molecular Cancer Therapeutics
Regulatory T cells (Treg) are known to suppress antitumor immune responses, and their presence in the tumor microenvironment is associated with cancer progression; therefore, Treg depletion is a promising strategy to enhance cancer immunotherapy. PF-08046032 is a novel antibody–drug conjugate (ADC) designed to target Tregs in the tumor microenvironment via CD25, the α-chain of the IL-2 receptor frequently upregulated by intratumoral Tregs. PF-08046032 is composed of an affinity-detuned anti-CD25 antibody linked to monomethyl auristatin E, a potent cytotoxic agent. Affinity detuning increases PF-08046032 selectivity for CD25high intratumoral Tregs while minimizing peripheral blood Treg depletion, thus reducing the risk of autoimmune toxicities. In preclinical experiments, PF-08046032 selectively depleted Tregs compared with CD8+ T cells and preferentially depleted Tregs with high CD25 expression. PF-08046032 showed dose-dependent antitumor activity in CD25-expressing human lymphoma xenograft models, whereas a similarly detuned anti-mouse CD25 surrogate ADC depleted intratumoral Tregs and drove CD8+ T-cell activation in murine tumor models. This effect resulted in robust antitumor activity as a single agent and in combination with anti-PD1 checkpoint inhibitor blockade. Lastly, PF-08046032 was well-tolerated in nonhuman primates and mitigated the persistent depletion of peripheral blood Treg that was observed with a high-affinity anti-CD25 ADC comparator, demonstrating the safety benefit of a detuned-affinity ADC format. PF-08046032 represents an innovative therapeutic approach for depletion of intratumoral Tregs that may offer an improved safety profile and efficacy over traditional Treg-depleting agents.
This article was featured on the cover of the July issue.

Journal: Cancer Research Communications
Cisplatin-Induced APE2 Overexpression Disrupts MYH9 Function and Causes Hearing Loss
Cisplatin remains a cornerstone chemotherapy for many solid tumors but is limited by dose-limiting toxicities, including nephrotoxicity, peripheral neuropathy, and ototoxicity—the latter of which disproportionately affects pediatric patients and lacks effective prevention strategies. Although therapeutic approaches to mitigate cisplatin-induced toxicity are urgently needed, the underlying mechanisms driving organ-specific injury remain incompletely understood. We previously identified apurinic/apyrimidinic endonuclease (APE) 2 as a critical mediator of cisplatin-induced acute kidney injury through disruption of mitochondrial integrity. In this study, we extend these findings to cisplatin-induced hearing loss (C-HL). We demonstrate that cisplatin selectively induces APE2, but not APE1, overexpression in murine and human outer hair cells. Using an inducible, outer hair cell–specific APE2 transgenic mouse model, we show that APE2 overexpression alone is sufficient to cause high-frequency hearing loss, accompanied by hair cell loss and stereocilia disorganization visualized by electron microscopy. Mechanistically, we identified a direct interaction between APE2 and MYH9, mapped the critical MYH9-binding domains, and demonstrated that APE2 knockdown preserved mitochondrial metabolism and protected cochlear cells from cisplatin-induced apoptosis. Notably, APE2 depletion activated an ATR–p53 signaling axis, promoting nuclear p53 localization and suppressing mitochondrial apoptotic pathways. Together, these findings reveal a noncanonical, APE2-dependent mechanism driving C-HL and suggest that targeting APE2 may offer a novel therapeutic strategy to prevent cisplatin-induced ototoxicity.
Significance: These results reveal an unexpected role of APE2 via its interaction with MYH9, emphasizing the therapeutic promise of targeting APE2 for preventing C-HL in patients with cancer.