AACR Journals Editors’ Picks for February
February is drawing to a close, and it’s time for our monthly staple: the collection of 10 “must read” articles hand-selected by the editors of the AACR journals. This edition features preclinical research focused on the rare cancer cholangiocarcinoma, results from clinical trials, and a look at medical financial hardship among cancer survivors, among other studies. As always, articles highlighted here are freely available for a limited time.
Journal: Molecular Cancer Therapeutics
The tyrosine kinase HER3, which is activated in many cancer types, has been shown to play a role in acquired resistance to HER2– and EGFR-targeted therapies. In its activated state, HER3 can form a heterodimer with either HER2 or EGFR, resulting in activation of the PI3K pathway, which can fuel uncontrolled cellular growth and proliferation. Previous antibodies against HER3 have been largely inefficacious, likely because they fail to block heterodimerization. In this study, the authors describe 10D1F, an anti-HER3 neutralizing antibody that binds to the heterodimerization interface. The researchers found that 10D1F could inhibit the formation of both EGFR:HER3 and HER2:HER3 heterodimers, resulting in decreased PI3K signaling in a variety of tumor models. Further, compared with other anti-HER3 antibodies, 10D1F demonstrated superior inhibition of tumor growth in multiple mouse xenograft models. The authors conclude that this novel antibody is a promising therapeutic candidate for the treatment of solid tumors driven by HER3. This article was highlighted in the February issue.
Journal: Cancer Research (February 15 issue)
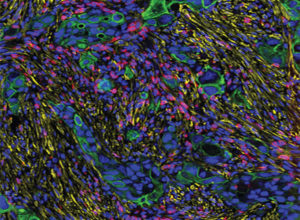
Cholangiocarcinoma, a rare cancer that forms in the bile ducts, is often diagnosed at an advanced stage, and the five-year survival for non-resectable disease is around 2 percent. While aberrant signaling of the Notch and Wnt pathways are observed in cholangiocarcinoma, how these pathways are initiated and maintained in this cancer type remains unknown. In this study, the authors report that the transcription factor proline-rich homeodomain protein (PRH), also known as hematopoietically expressed homeobox protein (HHEX), was highly expressed in patient samples of cholangiocarcinoma. Further, among mouse cholangiocarcinoma xenograft models, those that had a knockdown of PRH had significantly reduced tumor growth, and the overexpression of PRH in primary human biliary epithelial cells resulted in an invasive phenotype. Through functional enrichment analyses, the authors report that PRH regulates pathways associated with both EMT and Wnt signaling. Additionally, the researchers found that the knockdown of NOTCH3 decreased the expression of PRH, suggesting a positive transcriptional feedback loop. Treatment of cholangiocarcinoma cells with the CDK4/6 inhibitor palbociclib had strongly reduced proliferation in the presence of exogenous PRH. The authors conclude that monitoring PRH and NOTCH3 levels in individuals at high risk may help predict the development of cholangiocarcinoma, and that cholangiocarcinomas with high levels of PRH may be sensitive to CDK4/6 inhibitors. This article was highlighted and featured on the cover of the February 15 issue.
Journal: Cancer Prevention Research
Bisphosphonates (BPs), which are used to treat bone disorders, and metformin, which is used to treat diabetes, have been suggested to reduce cancer risk. However, the ability of these agents to reduce the risk for colorectal cancer, one of the leading causes of cancer-related death in the United States, has not been evaluated. Here, the authors used an azoxymethane-induced rat colon cancer model to assess the effect of two BPs (Zometa and Fosamax) and metformin on colorectal cancer risk. The drugs were evaluated individually and in combination (Zometa plus metformin or Fosamax plus metformin). Rats received the treatment through their diet, beginning four weeks after azoxymethane induction. Treatment with Zometa, Fosamax, or metformin alone had no significant effect on tumor incidence (percent of rats with colon tumors); however, combining metformin with either Zometa or Fosamax significantly reduced tumor incidence. Zometa or Fosamax alone significantly reduced the multiplicity (number of tumors of per rat) of non-invasive colon adenocarcinomas compared to the untreated control. Tumors from rats treated with the combinations had decreased expression of cell proliferation biomarkers and increased expression of apoptosis biomarkers. Together, the results demonstrate that combining metformin with either Zometa or Fosamax synergistically reduces the incidence of colon adenocarcinomas in a rat model of colon cancer. The authors propose that BPs may be potential chemopreventive agents for colorectal cancer. This article was highlighted in the February issue.
Journal: Cancer Immunology Research
The risk of colitis-associated colon cancer (CACC) is increased in patients with ulcerative colitis, a chronic intestinal inflammation disorder. Changes in the glycosylation of the MUC1 oncoprotein are common in chronic inflammatory conditions; abnormally glycosylated MUC1 promotes carcinogenesis. Here, the authors investigated the mechanism leading to abnormal MUC1 glycosylation. They found increased expression of inflammatory macrophage-associated cytokines in inflamed and malignant colon tissue compared with normal tissue. Consistent with this finding, the authors observed increased accumulation of macrophages in ulcerative colitis and CACC tissue samples. To investigate the impact of macrophage accumulation, the authors co-cultured macrophages with a colon cancer cell line. The ST6GALNAC1 glycosyltransferase, whose activity could lead to the shorter, tumor-associated glycosylation seen on MUC1, was found to be significantly upregulated in colon cancer cells co-cultured with M2 macrophages and in tissue samples of ulcerative colitis and CACC. Cytokine profiling revealed that co-culture of colon cancer cells with M2 macrophages led to increased expression of IL13 and CCL17. The inhibition of either cytokine significantly decreased the expression of ST6GALNAC1 in colon tumor cells, indicating that IL13 and CCL17 are involved in the observed upregulation of this glycosyltransferase. Further investigation demonstrated that IL13 and CCL17 promote ST6GALNAC1 expression through phosphorylation of STAT6 and p65, respectively. Together, the results from this study demonstrate that the crosstalk between colon cells and macrophages may affect glycosylation and promote pathogenesis of ulcerative colitis and CACC. This article was highlighted and was featured on the cover of the February issue.
Journal: Clinical Cancer Research (February 1 issue)
Treatment for patients with meningioma is often surgery followed by radiation therapy, and patients with aggressive disease can experience tumor recurrence, where treatment options are limited. In this phase II clinical trial, the combination of everolimus, an mTOR inhibitor, and octreotide, an agonist of the hormone somatostatin, was evaluated in 20 patients with aggressive, recurrent meningiomas. The overall six-month progression-free survival rate, overall six-month survival rate, and overall 12-month survival rate were 55 percent, 90 percent, and 75 percent, respectively. Further, 78 percent of tumors exhibited a major decrease in growth rate (greater than 50 percent) at three months. The authors conclude that this combinatorial treatment warrants further study, and that the exploratory endpoint of tumor growth rate should be considered in future clinical trials on meningiomas. This article was highlighted in the February 1 issue.
Journal: Clinical Cancer Research (February 15 issue)
Clear cell renal cell carcinoma, a rare type of kidney cancer, is often driven by overactivation of the transcription factor HIF-2. A phase I clinical trial evaluating the first-in-class HIF-2 inhibitor PT2385 in patients with advanced clear cell renal cell carcinoma previously found this treatment was safe and active, with 42 percent of patients achieving disease control for at least four months. In a prospective companion substudy, imaging and biomarker data were analyzed from 10 patients to elucidate the mechanism of PT2385 action. The researchers found that PT2385 inhibited HIF-2 in both tumor and nontumor tissues, and that the inhibitor was selective for HIF-2, as it did not affect HIF-1 complexes. Further, the authors report that prolonged treatment can result in resistance to PT2385, and that a gatekeeper mutation interferes with drug binding. These results suggest a fundamental dependency of clear cell renal cell carcinoma on HIF-2 for progression, which the authors note could be further exploited therapeutically. This article was highlighted in the February 15 issue.
Journal: Cancer Discovery
Histone Lysine Methylation Dynamics Control EGFR DNA Copy Number Amplification
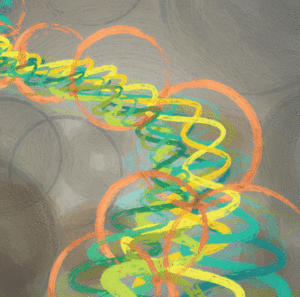
Many tumors exhibit amplification of oncogenes, such as MYC or EGFR; however, the underlying mechanisms are not clear. In this study, the authors investigated several histone lysine methyltransferases (KMTs) and demethylases (KDMs) to understand their contributions to amplification of EGFR. Using cancer cell lines, they found that interfering with H3K9 and H3K27 methylation promoted EGFR amplification. Contrastingly, both the overexpression of KDM4A in conjunction with certain KMTs (KMT2A/MLL1, SETD1A, and SETD1B) and the suppression of other KMTs, such as H3K9 KMTs and the H3K27 KMT EZH2, led to EGFR amplification. Consistent with these findings, treatment of cells with chemical inhibitors of KMTs and KDMs were found to impact EGFR copy number. Finally, the authors demonstrated that extrinsic cellular cues, such as hypoxia and the presence of epidermal growth factor, modulated the KMTs and KDMs that regulate EGFR copy number, leading to increased EGFR amplification. Together, the results from this study reveal the chromatin modifiers and extracellular signals that regulate EGFR amplification. The authors propose that epigenetic therapies could be used to modulate EGFR copy number in cancer. This article was highlighted and featured on the cover of the February issue.
Journal: Molecular Cancer Research
Mutations in PIK3CA, the catalytic subunit of PI3K, are observed in roughly 25 percent of patients with colorectal cancer and have been implicated in epithelial-to-mesenchymal transition (EMT), migration, and chemoresistance. How chromatin-modifying enzymes function in the context of PIK3CA mutations in colorectal cancer remains relatively unknown. In this study, the authors report that lysine-specific demethylase 1 (LSD1), a component of the transcription-repressing CoREST complex, is significantly overexpressed in patients with colorectal cancer harboring mutations in PIK3CA compared with patients with colorectal cancer with wild type PIK3CA. Studies conducted in colorectal cancer cell lines revealed that LSD1 enhances the activation of the AKT kinase, and that the genetic disruption of LSD1 blocks AKT-mediated EMT and migration when PIK3CA mutations are present. The authors conclude that inhibitors of the CoREST complex may represent a potential therapeutic strategy for patients with PIK3CA-mutant colorectal cancer. This article was highlighted and featured on the cover of the February issue.
Journal: Cancer Epidemiology, Biomarkers, and Prevention
The costs of cancer care are rising, leading to a greater risk of medical financial hardship. In this study, the authors examined the prevalence of medical financial hardship and the factors that contribute to it. The authors identified 963 cancer survivors from the 2016 Medical Expenditures Panel Survey – Experiences with Cancer and measured three different domains of medical financial hardship: material hardship (e.g. filing for bankruptcy), psychological hardship (e.g. worrying about finances), and behavioral hardship (e.g. delaying or forgoing cancer care due to cost). They found that 53.6 percent of cancer survivors between the ages of 18 and 64 years experienced at least one hardship domain, 28.4 percent experienced at least two domains, and 11.4 percent experienced all three domains. Among those over 65 years, 42 percent experienced at least one domain, 12.7 percent experienced at least two domains, and 4 percent experienced all three domains. Survivors under 65 years of age were significantly more likely than those over 65 years to have made financial sacrifices due to their cancer. Factors associated with increased hardship included low income, lower education level, being a racial/ethnic minority, comorbidity, lack of private insurance coverage, extended employment change, and recent cancer treatment. The authors conclude that medical financial hardship is substantial among cancer survivors, particularly among younger survivors. This article was highlighted in the February issue.
Journal: Cancer Research (February 1 issue)
Monitoring disease progression can be challenging due to the inaccessibility of certain locations in organs. Here, the authors investigated the potential of using an implanted synthetic biomaterial scaffold to monitor disease progression and recurrence. Mice received implants of synthetic scaffolds and were then inoculated with 4T1 tumor cells. Biopsied tissue from the implants underwent genetic analyses, which identified gene expression changes in myeloid cells that correlated with disease progression. Similar gene expression patterns were observed in diseased lung tissue – the usual primary metastatic site for 4T1 cells – suggesting that the gene expression patterns observed in the implant reflect those seen in natural metastatic sites. The authors then investigated the utility of the implant to predict outcomes by monitoring recurrence after treatment. Following excision of the primary tumor, gene expression in the biopsied implant tissue correlated with disease recurrence. Together, the results indicate that gene expression changes within an implanted synthetic biomaterial scaffold can reflect disease progression and recurrence. This article was accompanied by a related commentary and was featured on the cover of the February 1 issue.


