Tarlatamab-dlle Approved for Metastatic Lung Cancer
Tarlatamab-dlle is the first t-cell engager that uses the bispecific antibody design for a solid tumor. The U.S. Food...


Tarlatamab-dlle is the first t-cell engager that uses the bispecific antibody design for a solid tumor. The U.S. Food...
The CAR T-cell therapy received accelerated approval to treat advanced cases of pretreated follicular lymphoma. The U.S. Food and...

Tisotumab vedotin-tftv is approved to treat certain recurrent or metastatic cervical cancers. The U.S. Food and Drug Administration (FDA)...
The FDA approved the BRAF inhibitor tovorafenib for pediatric patients with certain Genetic alterations. The U.S. Food and Drug...
The FDA has approved lutetium Lu 177 dotatate for children 12 years or older with some gastroenteropancreatic neuroendocrine tumors. ...
The FDA has approved nogapendekin alfa inbakicept-pmln, which stimulates immune cells to fight bladder cancer. The U.S. Food and...
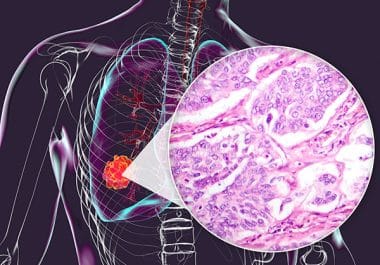
The FDA has approved the ALK inhibitor alectinib for surgically resectable NSCLC. The U.S. Food and Drug Administration (FDA)...
Fam-trastuzumab deruxtecan-nxki was approved for HER2-positive tumors, regardless of the tissue of origin. The U.S. Food and Drug Administration...
Mirvetuximab soravtansine-gynx was approved for certain ovarian, fallopian tube, and peritoneal cancers. The U.S. Food and Drug Administration (FDA)...
The BCR-ABL inhibitor ponatinib was approved for some patients with acute lymphoblastic leukemia. The U.S. Food and Drug Administration...