Erdafitinib Granted Full Approval for Bladder Cancer
The FDA has approved the FGFR inhibitor erdafitinib for certain patients with urothelial carcinoma The U.S. Food and Drug...


The FDA has approved the FGFR inhibitor erdafitinib for certain patients with urothelial carcinoma The U.S. Food and Drug...

The FDA expanded the indications for pembrolizumab to include more patients with stage III-IVA cervical cancer. The U.S. Food...
Enfortumab vedotin-ejfv plus pembrolizumab was approved for certain bladder cancers. The U.S. Food and Drug Administration (FDA) has approved...
Belzutifan is now available for certain patients with kidney cancer whether or not they have von Hippel-Lindau disease. The...
The FDA has approved eflornithine to prevent relapse after standard-of-care therapy for neuroblastoma. The U.S. Food and Drug Administration...
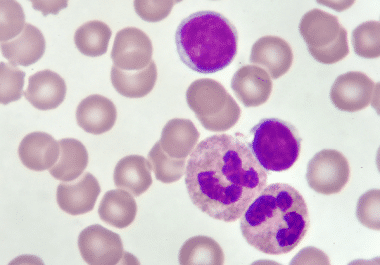
The FDA has approved pirtobrutinib for patients with heavily pretreated chronic lymphocytic leukemia or small lymphocytic lymphoma. The U.S....
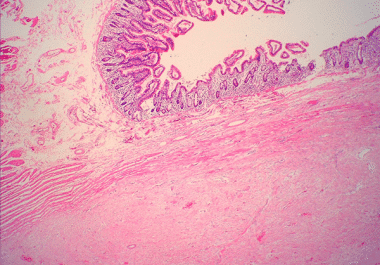
The FDA has approved nirogacestat for the treatment of noncancerous but painful growths called desmoid tumors. The U.S. Food...
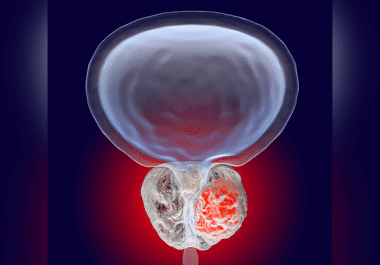
The FDA has approved enzalutamide for non-metastatic prostate cancer that is sensitive to antiandrogen therapy. The U.S. Food and...
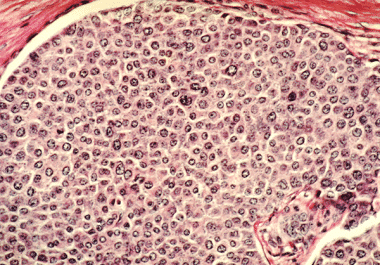
The FDA has approved capivasertib for the treatment of breast cancers with certain mutations. The U.S. Food and Drug...
The FDA has approved pembrolizumab for the first-line treatment of certain HER2-negative gastric and gastroesophageal junction cancers. The U.S....