Updated Indications for Brain Cancer Chemotherapy
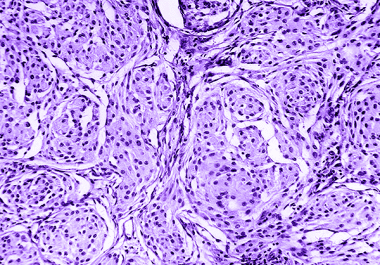
The FDA Project Renewal initiative has updated the approved indications for temozolomide chemotherapy in anaplastic astrocytoma. The U.S. Food...


The FDA Project Renewal initiative has updated the approved indications for temozolomide chemotherapy in anaplastic astrocytoma. The U.S. Food...

The FDA approved a kit that allows for liver-directed delivery of the chemotherapy melphalan to treat metastatic liver disease...
The FDA has approved another T-cell engager therapy for patients with heavily pretreated multiple myeloma The U.S. Food and...
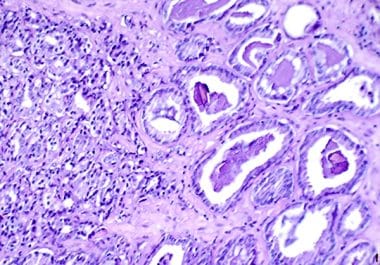
The FDA approved a combination of the PARP inhibitor niraparib with a hormone therapy agent for certain patients with...
The FDA granted full approval to pralsetinib as a treatment for certain patients with non-small cell lung cancer The...
Certain patients with advanced multiple myeloma may be eligible for treatment with a new immunotherapy drug. The U.S. Food...

The FDA approved a combination of trifluridine, tipiracil, and bevacizumab for the treatment of heavily pretreated metastatic colorectal cancer. ...

The FDA has approved an immune checkpoint inhibitor in combination with chemotherapy to treat certain adult patients with endometrial...
The FDA has approved an inhibitor of FLT3 for use across multiple phases of acute myeloid leukemia treatment. The...

New oral hormonal therapy offers alternative to injections for some women with metastatic disease.