Expanded Indication for a Targeted Therapy for Early Breast Cancer
The FDA approved abemaciclib with endocrine therapy for HR-positive, HER2-negative, early-stage breast cancer at high risk of recurrence. The...


The FDA approved abemaciclib with endocrine therapy for HR-positive, HER2-negative, early-stage breast cancer at high risk of recurrence. The...
A drug that selectively targets cancer cells was approved for the treatment of certain HR-positive breast cancers. The U.S....
The FDA approved the immune checkpoint inhibitor dostarlimab-gxly to treat advanced uterine cancer with certain mutations. The U.S. Food...
The FDA has approved the oral SERD elacestrant to treat certain patients with estrogen receptor-positive breast cancer. The U.S....
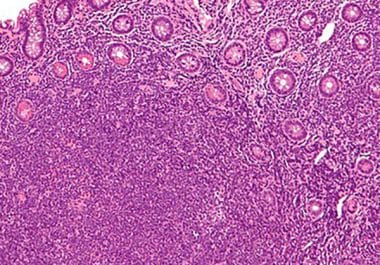
The FDA granted accelerated approval to the BTK inhibitor pirtobrutinib for the treatment of mantle cell lymphoma. The U.S....
Pembrolizumab was approved to treat some patients with lung cancer after surgery and chemotherapy. The U.S. Food and Drug...

The FDA approved tucatinib for certain patients with unresectable or metastatic colorectal cancer. The U.S. Food and Drug Administration...
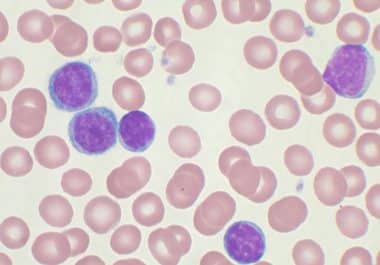
The FDA approved zanubrutinib, a second-generation Bruton’s tyrosine kinase inhibitor. The U.S. Food and Drug Administration (FDA) has approved...
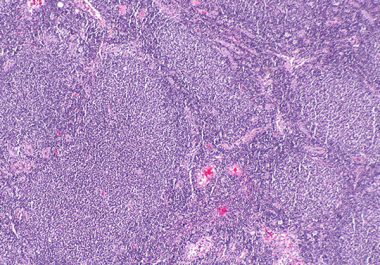
The FDA granted accelerated approval to mosunetuzumab-axgb, the first bispecific antibody approved to treat a form of non-Hodgkin lymphoma. ...
The FDA approved the adenovirus-based gene therapy nadofaragene firadenovec for the treatment of localized bladder cancer. The U.S. Food...