Targeting a Gene Fusion in Solid Tumors
The FDA granted accelerated approval to selpercatinib to treat solid tumors harboring RET gene fusions. The U.S. Food and...


The FDA granted accelerated approval to selpercatinib to treat solid tumors harboring RET gene fusions. The U.S. Food and...

The FDA granted full approval to selpercatinib for the treatment of patients with advanced lung cancer. The U.S. Food...
An inhibitor of the growth-stimulating FGFR1 protein was approved for patients with a rare type of blood cancer. The...
The FDA granted accelerated approval to the first HER2-targeted therapy for the treatment of non-small cell lung cancer with...

The FDA approved the kinase inhibitor capmatinib for lung cancers harboring a certain mutation in the protein MET. The...
The FDA approved a HER2-targeted therapy for the treatment of breast tumors with low HER2 expression. The U.S. Food...
The FDA approved darolutamide for the treatment of metastatic prostate cancer that is sensitive to hormone-blocking treatment. The U.S....
The FDA approved a kinase inhibitor for the treatment of inflammatory myofibroblastic tumors in children and adults. The U.S....
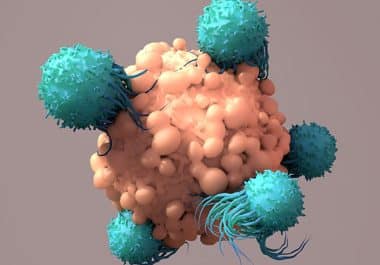
The CAR T-cell therapy lisocabtagene maraleucel was approved for the second-line treatment of certain patients with large B-cell lymphoma. ...

Ibrutinib, a small molecule inhibitor of B-cell proliferation, was approved for pediatric patients with chronic graft-versus-host disease. The U.S....