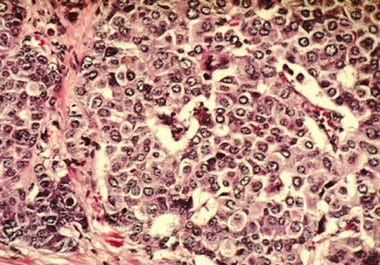
PARP Inhibitor Approved as Adjuvant Treatment for High-risk, Early Breast Cancer
The FDA approved olaparib for patients with breast cancer who have inherited BRCA mutations and have received prior neoadjuvant...


The FDA approved olaparib for patients with breast cancer who have inherited BRCA mutations and have received prior neoadjuvant...
The FDA approved nivolumab in combination with chemotherapy for use before surgery in certain patients with non-small cell lung...
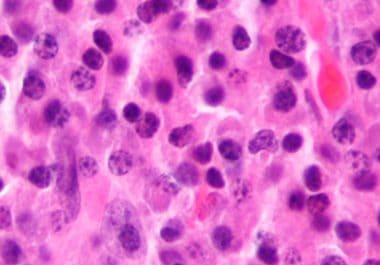
The FDA recently approved the second cellular immunotherapy to treat certain adult patients with multiple myeloma. The U.S. Food...
The FDA approved the targeted immunotherapy tebentafusp-tebn for certain adult patients with uveal melanoma. The U.S. Food and Drug...
The FDA approved an immune checkpoint inhibitor for patients with stage IIB or IIC melanoma following surgical removal of...
The FDA approved a monoclonal antibody combined with chemotherapy TO TREAT certain childhood blood cancers that express the protein...
The U.S. FDA approved a new combination of existing drugs to treat certain patients with multiple myeloma The U.S....
A new drug approved by the U.S. FDA may help surgeons find cancer more easily and reduce the likelihood...
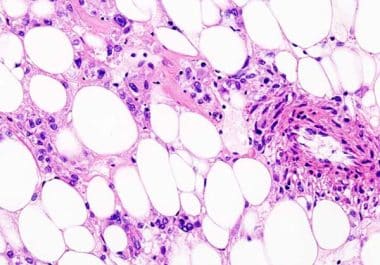
The U.S. FDA approved the first treatment for malignant perivascular epithelioid cell tumor The U.S. Food and Drug Administration...
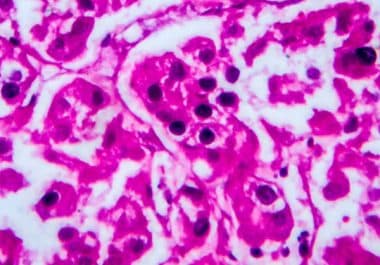
The FDA has approved an immunotherapy known as immune checkpoint inhibition for the treatment of certain patients with kidney...