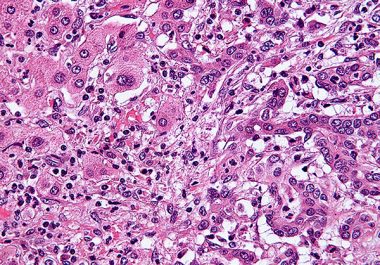
Targeting Bile Duct Cancer
The U.S. FDA approved a molecularly targeted therapeutic to treat certain patients with cholangiocarcinoma. The U.S. Food and Drug...


The U.S. FDA approved a molecularly targeted therapeutic to treat certain patients with cholangiocarcinoma. The U.S. Food and Drug...
The U.S. FDA granted accelerated approval to an immunotherapeutic and a companion diagnostic test to treat certain cancer patients...

The U.S. FDA approved a molecularly targeted therapeutic for certain patients with tumors associated with a raRe genetic disorder—von...
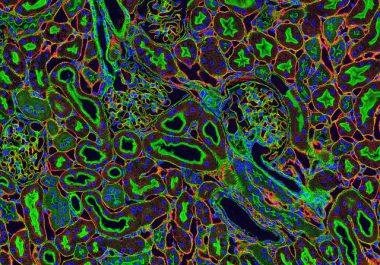
The U.S. FDA approved the combination of lenvatinib and pembrolizumab to treat adult patients with renal cell carcinoma, the...
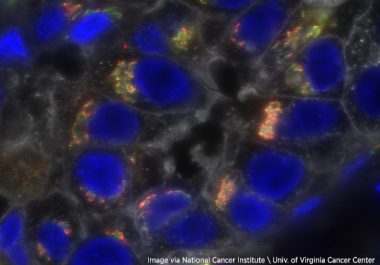
The U.S. FDA approved the immune checkpoint inhibitor pembrolizumab combined with chemotherapy to treat certain breast cancer patients before...
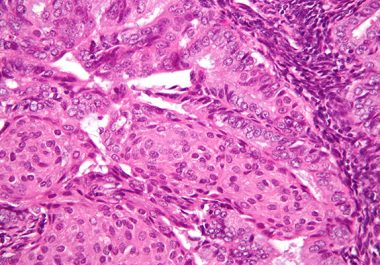
The U.S. FDA approved a combination of a molecularly targeted therapeutic and an immunotherapeutic to treat certain patients with...
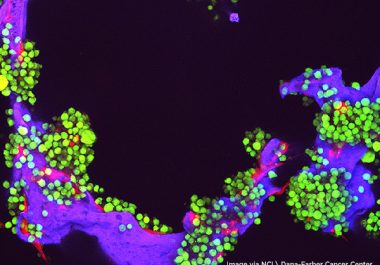
The U.S. FDA approved a monoclonal antibody and enzyme combination for use in conjunction with chemotherapy and a steroid...
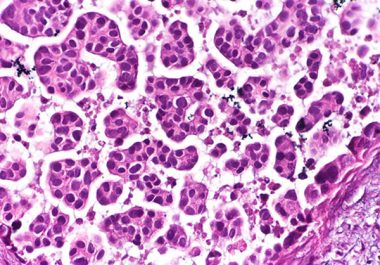
The U.S. FDA approved an antibody-drug conjugate to treat urothelial cancer, the most common form of bladder cancer. The...
The U.S. FDA approved a new chemotherapy as part of a multi-drug regimen for certain patients with two similar...
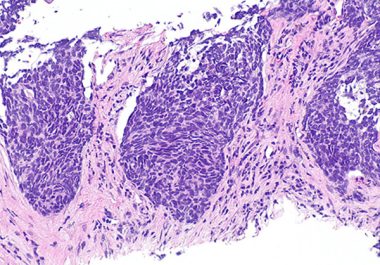
The U.S. FDA has granted accelerated approval to the first KRAS inhibitor, along with two companion diagnostics, for certain...