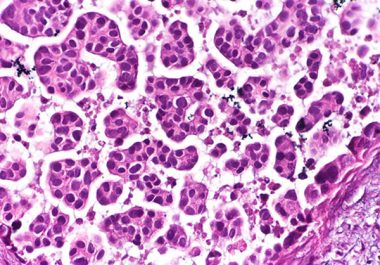
A New Therapy for Bladder Cancer
The FDA granted accelerated approval to the targeted therapeutic sacituzumab govitecan to treat certain patients with the most common...


The FDA granted accelerated approval to the targeted therapeutic sacituzumab govitecan to treat certain patients with the most common...
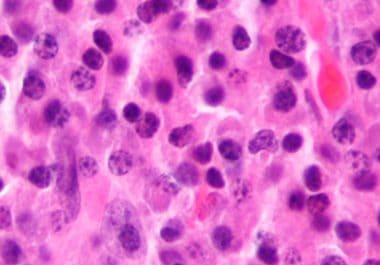
The FDA approved the first CAR T-cell therapy to treat certain adult patients with multiple myeloma. The U.S. Food...
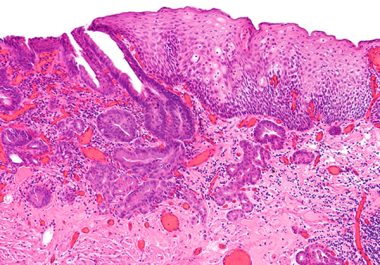
the immunotherapeutic pembrolizumab in combination with chemotherapy WAS APPROVED to treat certain adult patients with esophageal cancer. The U.S....
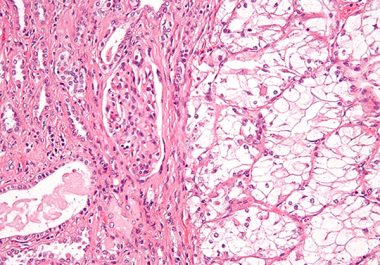
The FDA approved the molecularly targeted therapeutic tivozanib to treat certain adult patients with the most common type of...
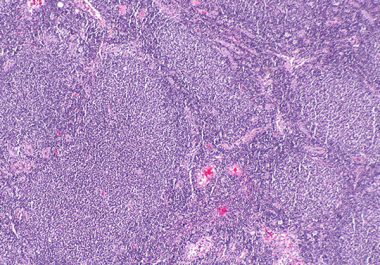
The FDA granted accelerated approval to a CAR T-cell therapy for certain adult patients with follicular lymphoma, a common...
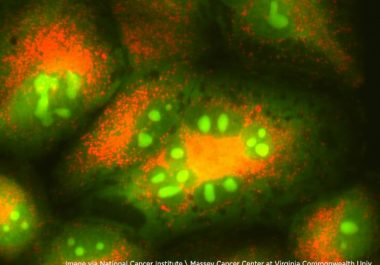
The FDA has approved an immune checkpoint inhibitor – a type of cancer immunotherapy – as a first-line therapeutic...
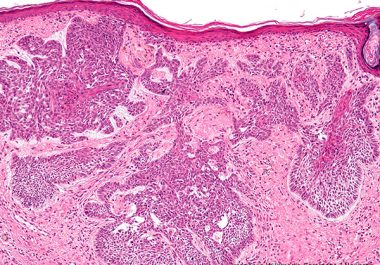
The FDA approved the immune checkpoint inhibitor, cemiplimab-rwlc, for certain patients with locally advanced or metastatic basal cell carcinoma—the...
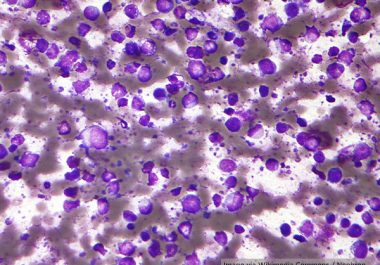
The FDA approved a CAR T-cell therapy – a class of cancer immunotherapy – to treat certain patients with...
The FDA granted accelerated approval to the molecularly targeted therapeutic umbralisib for certain patients with marginal zone lymphoma or...
The FDA granted accelerated approval to a new oral targeted therapeutic to treat certain adult patients with the most...