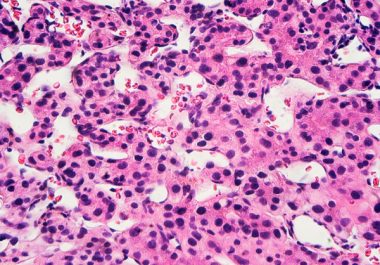
Aiming at Two Protein Targets to Treat Liver Cancer
The FDA approved a combination of an immunotherapy and a therapeutic that can stop tumors from growing blood vessels...


The FDA approved a combination of an immunotherapy and a therapeutic that can stop tumors from growing blood vessels...
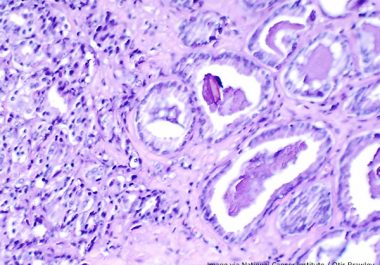
The FDA has expanded the use of the PARP-targeted therapeutic olaparib to include the treatment of certain patients with...
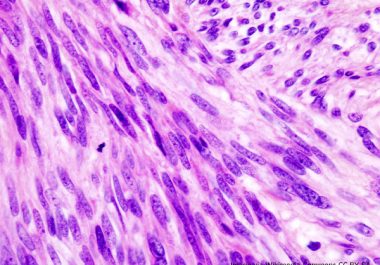
The FDA has approved a new molecularly targeted therapeutic for treating certain patients who have advanced gastrointestinal stromal tumors. The...

The FDA has expanded the use of a molecularly targeted therapeutic to include the treatment of certain patients with...
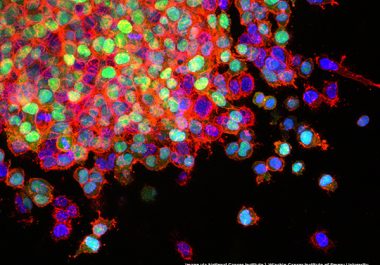
The FDA has approved a combination of nivolumab and ipilimumab to treat certain patients with metastatic lung cancer. The...
The FDA has expanded the use of an immunomodulatory therapeutic to include the treatment of adults who have Kaposi...

Over the course of the two-day AACR Virtual Annual Meeting I, more than 61,000 people from 140 countries around the...
The FDA has approved an alternative dosing schedule for the immune checkpoint inhibitor pembrolizumab. On April 28, 2020, the...
The FDA approved a new molecularly targeted therapeutic for certain patients with lung cancer driven by a mutation in...
The FDA has approved a type of molecularly targeted therapeutic called an antibody-drug conjugate for the treatment of metastatic...