Targeting a Group of Rare Tumors
The molecularly targeted therapeutic pexidartinib is the first ever systemic treatment approved by the FDA for the treatment of...

The molecularly targeted therapeutic pexidartinib is the first ever systemic treatment approved by the FDA for the treatment of...
The FDA has expanded the use of the immunotherapeutic pembrolizumab to treat certain patients with esophageal cancer. The U.S....
A new antihormone agent darolutamide (Nubeqa) provides men with nonmetastatic prostate cancer a third option to control the disease...
The newly approved therapeutic is for patients with multiple myeloma that has either stopped responding to or has failed...
The FDA has expanded the use of the immunotherapeutic pembrolizumab to treat certain patients with small cell lung cancer....
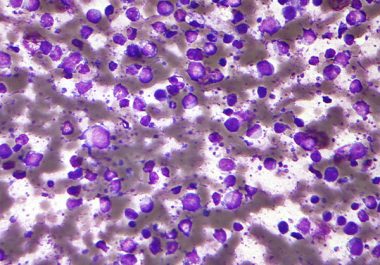
The molecularly targeted therapeutic polatuzumab vedotin-piiq is the first antibody-drug conjugate approved for treating diffuse large B-cell lymphoma. The...
The FDA has approved a combination of pembrolizumab and axitinib for treating certain patients with renal cell carcinoma, the...
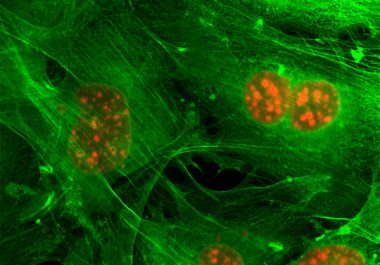
The molecularly targeted therapeutic alpelisib was approved for certain patients who have advanced or metastatic breast cancer. The U.S....
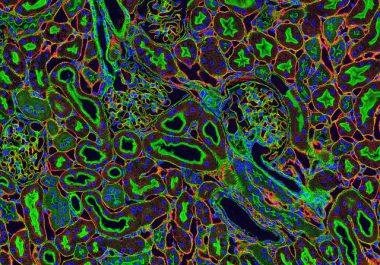
The approval provides a third immunotherapy-based combination treatment for patients newly diagnosed with advanced renal cell carcinoma, the most...
The first FGFR-targeted anticancer therapeutic to be approved by the FDA, erdafitinib, is intended for use in the treatment...