Combining Targeted Therapy and Immunotherapy for Non-Hodgkin Lymphoma
The FDA has approved the combination of ibrutinib and rituximab as a new treatment for a rare form of...

The FDA has approved the combination of ibrutinib and rituximab as a new treatment for a rare form of...

The FDA has approved a new targeted radiotherapeutic for treating two rare neuroendocrine tumors, pheochromocytomas and paragangliomas. The U.S....
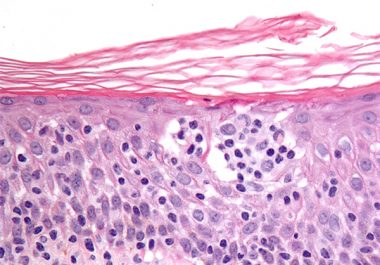
The FDA has approved a new immunotherapeutic for treating two types of cutaneous T-cell lymphoma, mycosis fungoides and Sézary...
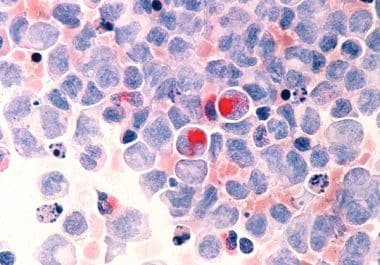
The new therapeutic was approved by the FDA for treating patients whose leukemia tests positive for a mutation in...
The combination of ipilimumab and nivolumab was approved by the FDA for metastatic colorectal cancer that is positive for...
The FDA has approved a third combination of targeted therapeutics for treating patients who have metastatic melanoma with certain...
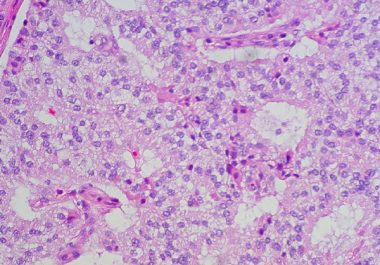
The FDA has approved a targeted radiotherapeutic for treating neuroendocrine tumors arising in the pancreas and different parts of...
The FDA has expanded the use of the immunotherapeutic pembrolizumab to treat certain patients with cervical cancer. The U.S....
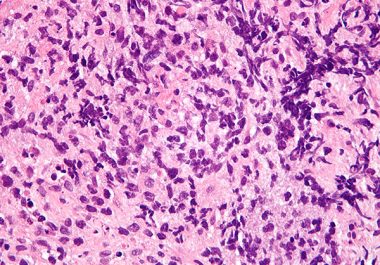
The FDA has expanded the use of pembrolizumab to treat certain patients with non-Hodgkin lymphoma. The U.S. Food and...
The FDA decision means tisagenlecleucel (Kymriah) is now approved for certain types of non-Hodgkin lymphoma and acute lymphoblastic leukemia....