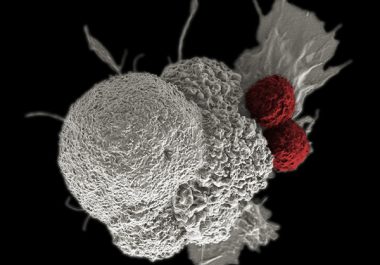
Expanding the Use of an Immunotherapeutic to Liver Cancer
The FDA decision means the immune checkpoint inhibitor nivolumab is now approved for eight types of cancer. Nivolumab (Opdivo)...


The FDA decision means the immune checkpoint inhibitor nivolumab is now approved for eight types of cancer. Nivolumab (Opdivo)...
The FDA has approved pembrolizumab for treating certain patients with advanced stomach (gastric) cancer. Use of the immunotherapeutic pembrolizumab...
A new molecularly targeted therapeutic to treat certain patients with follicular lymphoma, a common type of non-Hodgkin lymphoma, has...
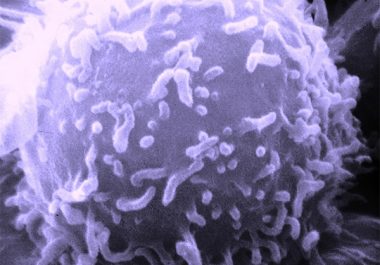
The FDA has approved a CD22-targeted antibody-drug conjugate for treating certain adults with ALL. The U.S. Food and Drug...
The new treatment, Vyxeos, comprises a combination of two commonly used cytotoxic chemotherapeutics inside a nanosized particle. The U.S....

Enasidenib is the second molecularly targeted therapeutic approved by the FDA for treating AML in recent months. The U.S....
The FDA decision means the immune checkpoint inhibitor nivolumab is now approved for seven types of cancer. Use of...
The U.S. Food and Drug Administration has approved the first of a new type of immunotherapy called CAR T-cell...
The FDA has approved a new HER2-targeted therapeutic for the treatment of certain patients with early-stage breast cancer. The...
The FDA’s recent announcement means the immune checkpoint inhibitor pembrolizumab is now approved for five forms of cancer. The...