Targeting Tumors Based on a Biomarker
The FDA’s new approval for the immunotherapeutic pembrolizumab is the first based entirely on a tumor biomarker rather than...


The FDA’s new approval for the immunotherapeutic pembrolizumab is the first based entirely on a tumor biomarker rather than...
Avelumab is the fourth immune checkpoint inhibitor to get FDA approval to treat certain patients with bladder cancer. In...
The FDA has approved a new immune checkpoint inhibitor, durvalumab, for the treatment of certain bladder cancer patients. The...
The approval expands the use of the anticancer therapeutic regorafenib (Stivarga) to a third cancer type. It is the...

The U.S. Food and Drug Administration approval of niraparib means there are now three PARP inhibitors that can be...

The U.S. Food and Drug Administration decision makes avelumab the first-ever approved treatment for metastatic Merkel cell carcinoma. The...
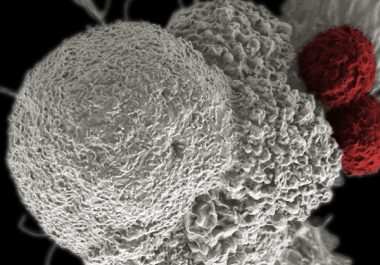
The FDA has approved the immunotherapeutic pembrolizumab to treat Hodgkin lymphoma. The U.S. Food and Drug Administration (FDA) recently...
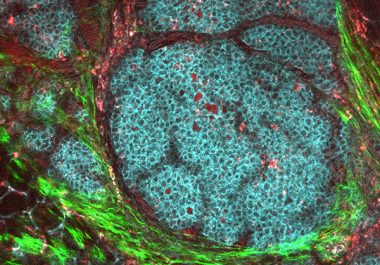
The FDA approved a molecularly targeted therapeutic for postmenopausal women with HR-positive, HER2-negative breast cancer. The U.S. Food and...
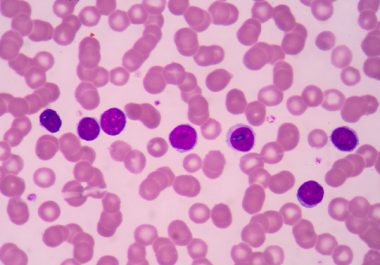
The U.S. Food and Drug Administration action means ibrutinib is now an approved treatment for three types of non-Hodgkin...
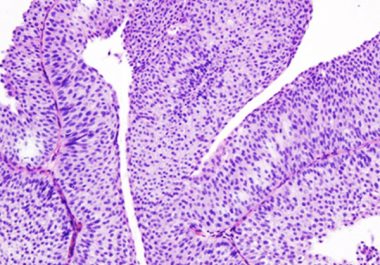
The U.S. Food and Drug Administration decision means nivolumab is now an approved treatment for six types of cancer....