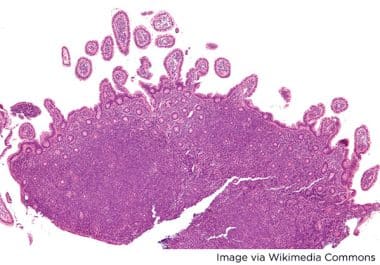
New Approvals for Acalabrutinib in Mantle Cell Lymphoma
Acalabrutinib was granted traditional approval as a monotherapy for previously treated MCL and as part of a combination for...


Acalabrutinib was granted traditional approval as a monotherapy for previously treated MCL and as part of a combination for...

The FDA approved nivolumab and hyaluronidase-nvhy as an injectable therapy for multiple tumor types. The U.S. Food and Drug...

The FDA approved the kinase inhibitors encorafenib and cetuximab plus chemotherapy for the treatment of certain colorectal cancers. The...

The FDA approved the mesenchymal stromal cell therapy remestemcel-L-rknd for some pediatric patients as young as 2 months. ...
The FDA approved ensartinib for certain patients with locally advanced or metastatic non-small cell lung cancer. The U.S. Food...
The FDA approved cosibelimab-ipdl for certain skin cancers that cannot be treated with surgery or radiation. The U.S. Food...
The FDA approved durvalumab for some patients with previously treated limited-stage small cell lung cancer. The U.S. Food...
The FDA approved the targeted therapy zenocutuzumab-zbco for certain patients with lung or pancreatic cancer. The U.S. Food and...
The FDA granted accelerated approval to the first-in-class HER2-directed bispecific antibody zanidatamab-hrii. The U.S. Food and Drug Administration (FDA)...

The FDA Project Renewal initiative has updated the approved indications for fludarabine phosphate chemotherapy in chronic lymphocytic leukemia. The...