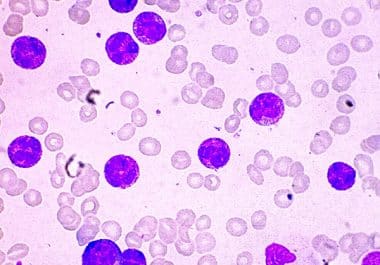
First Menin Inhibitor Approved for Certain Acute Leukemias
The FDA approved revumenib, a first-in-class menin inhibitor, to treat acute leukemias with a KMT2A translocation. The U.S. Food...


The FDA approved revumenib, a first-in-class menin inhibitor, to treat acute leukemias with a KMT2A translocation. The U.S. Food...
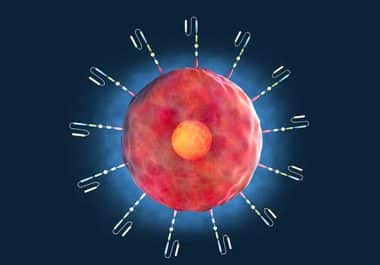
The cell-based immunotherapy was approved for adults with relapsed or refractory acute lymphoblastic leukemia. The U.S. Food and Drug...
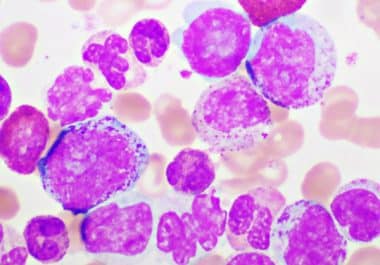
The therapeutic was approved for certain patients with Philadelphia chromosome-positive chronic myeloid leukemia. The U.S. Food and Drug Administration...
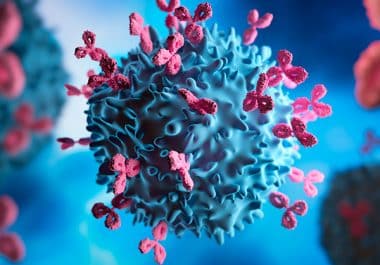
The FDA approved the claudin 18.2-targeted therapy Zolbetuximab-clzb for some gastric and gastroesophageal junction cancers. The U.S. Food and Drug...

The PI3K inhibitor is approved as part of combination therapy for certain patients whose breast cancer recurred on or...
The immunotherapy is now approved to treat certain lung cancers before and after surgery. The U.S. Food and Drug...

The accelerated approval of selpercatinib for certain patients with thyroid cancer was converted to a full approval. The U.S....
The EGFR inhibitor osimertinib is now available as a monotherapy for certain patients with locally advanced, unresectable non-small cell...
The CD38-targeted antibody was approved for patients who are ineligible for stem cell transplant. The U.S. Food and Drug...
Amivantamab with carboplatin and pemetrexed was approved for some advanced non-small cell lung cancers that progressed on or after...