An Immunotherapeutic For Multiple Myeloma
Daratumumab is the first monoclonal antibody approved by the U.S. Food and Drug Administration for patients with multiple myeloma
The U.S. Food and Drug Administration (FDA) has approved the immunotherapeutic daratumumab (Darzalex) for treating certain patients with multiple myeloma.
Daratumumab is intended for patients whose multiple myeloma has worsened despite receiving at least three other treatments for their disease. The new immunotherapeutic was shown to reduce tumor burden for patients in two early-stage clinical trials.
Multiple myeloma is one of the most commonly diagnosed hematological malignancies – blood cancers. In fact, the National Cancer Institute estimates that there will be 26,850 new cases of multiple myeloma and 11,240 multiple myeloma-related deaths in the United States this year.
New therapeutics for multiple myeloma are urgently needed. Despite the development and FDA approval of new therapeutics – including proteasome inhibitors like bortezomib (Velcade) and carfilzomib (Kyprolis), and immunomodulatory agents like lenalidomide (Revlimid) and pomalidomide (Pomalyst) – which have improved patient outcomes in recent years the overall five-year survival rate remains under 50 percent.
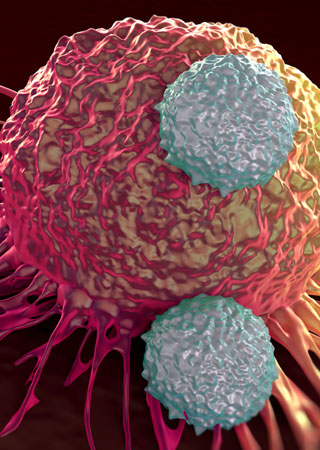
Daratumumab is a monoclonal antibody that attaches to a protein called CD38, which is found at high levels on the surface of myeloma cells. It exerts its anticancer effects in a number of ways, including by flagging the myeloma cells for several types of immune cells. Once the immune cells have been directed to their target, they attack and destroy the myeloma cells.
According to the FDA, the agency approved daratumumab based on results from two clinical trials. In the first of these, the phase II SIRIUS trial, 29 percent of the 106 participants had a complete or partial reduction in their tumor burden – that is to say 29 percent had an objective response – and the median duration of the responses was 7.4 months. Results from the second trial, which were published recently in The New England Journal of Medicine, showed that the objective response rate among the 42 participants who received the dose of daratumumab that the FDA ultimately approved was 36 percent.
Because this approval was based on objective response rate data, rather than overall survival, daratumumab’s manufacturer, Janssen, is required by the FDA to conduct a study to confirm that the drug improves survival for patients.
The FDA approval of daratumumab is specifically for patients whose multiple myeloma has progressed despite treatment with at least three prior forms of therapy, including a proteasome inhibitor and an immunomodulatory agent, as well as those whose disease is not responsive to either a proteasome inhibitor or an immunomodulatory agent. This approval helps address a clear unmet need because these patients have a very poor prognosis, with median overall survival estimated to be about nine months.
The FDA approval was rendered on Nov. 16, 2015.
