A New Immunotherapy for Multiple Myeloma
Elotuzumab became the third new treatment for multiple myeloma approved by the U.S. Food and Drug Administration in a two-week period
The U.S. Food and Drug Administration (FDA) approved the immunotherapeutic elotuzumab (Empliciti) for treating certain patients with multiple myeloma.
Elotuzumab is intended for use in combination with lenalidomide (Revlimid) and dexamethasone for treating patients who have multiple myeloma that has worsened despite treatment with one to three other medications. The three-drug combination increased the time before disease progressed for patients in a randomized phase III clinical trial.
In November 2015, the FDA approved two other therapeutics for treating certain patients with multiple myeloma: daratumumab (Darzalex) and ixazomib (Ninlaro).
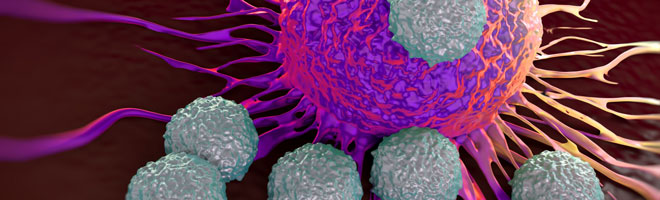
Elotuzumab is unique among the three new multiple myeloma treatments in the way it exerts its anticancer effects. It is a monoclonal antibody that attaches to a protein called SLAM7F, which is found at high levels on the surface of both myeloma cells and immune cells called natural killer cells. Differences in these two cell types mean elotuzumab has distinct effects on them when it attaches to SLAM7F on their surfaces, and these effects provide a two-pronged attack on multiple myeloma. Specifically, it directly activates natural killer cells, enhancing their ability to kill myeloma cells, and it flags myeloma cells for a number of immune cell types, which, once directed to the myeloma cells, attack and destroy them.
The FDA approval of elotuzumab was based on results from the phase III ELOQUENT-2 clinical trial, which were published recently in The New England Journal of Medicine. Specifically, the decision was made because adding elotuzumab to lenalidomide and dexamethasone increased the time before disease progressed by four and a half months and because it increased by 13 percentage points (from 65.5 percent to 78.5 percent) the proportion of patients who had complete or partial shrinkage of their tumors.
The three newly approved multiple myeloma treatments represent important advances against a disease that is anticipated to be diagnosed in 26,850 people in the United States this year alone. They also provide new hope for patients who have been living with the disease for a number of years, like Cindy Chmielewski, who was diagnosed with multiple myeloma in 2006.
Learn more about Cindy’s journey and how she uses social media to help others in this video:
The FDA approval was rendered on Nov. 20, 2015.
