
Targeting Radiotherapy to Treat Rare Neuroendocrine Tumors
The FDA approved a targeted radiotherapeutic for treating certain patients with two types of neuroendocrine tumors.


The FDA approved a targeted radiotherapeutic for treating certain patients with two types of neuroendocrine tumors.
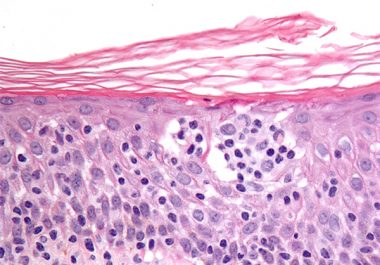
The FDA approved a new immunotherapeutic for treating certain patients with two rare types of non-Hodgkin lymphoma.
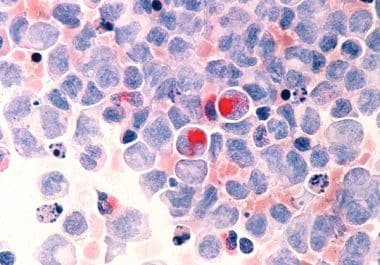
The FDA approved a new molecularly targeted therapeutic for treating certain patients with acute myeloid leukemia (AML).
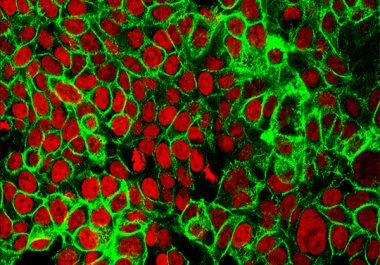
The FDA approved a combination of two immunotherapeutics for treating patients with colorectal cancer.
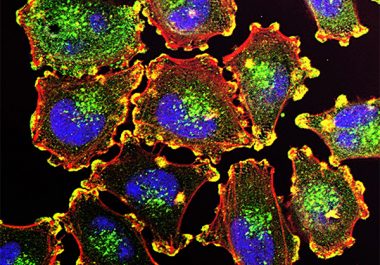
The FDA approved two molecularly targeted therapeutics for use in combination to treat patients with metastatic melanoma.
The FDA approved a new anticancer therapeutic for treating certain patients with neuroendocrine tumors.

Breast cancer is the most commonly diagnosed cancer in the United States. In 2018, 266,120 women and 2,550 men are expected to receive the news that they have the disease, according to National Cancer Institute data.

Following dietary recommendations may reduce the risk of several cancers, a study in the AACR journal Cancer Research reports. Learn more.

The FDA approved pembrolizumab for patients with recurrent or metastatic cervical cancer that tests positive for PD-L1 and that has progressed despite chemotherapy.
The FDA expanded the use of the immunotherapy pembrolizumab for certain patients with an aggressive type of non-Hodgkin lymphoma.