Expanding Molecularly Targeted Therapy to Additional Types of Lymphoma
The FDA expanded the use of a targeted therapy to include certain patients with two types of non-Hodgkin lymphoma.


The FDA expanded the use of a targeted therapy to include certain patients with two types of non-Hodgkin lymphoma.
The FDA approved a molecularly targeted therapeutic for adults with mantle cell lymphoma that has progressed despite at least one prior treatment.
The FDA approved the second of a revolutionary new type of immunotherapy known as chimeric antigen receptor (CAR) T-cell therapy.
Mexican-Americans face different – and perhaps greater – risks for developing liver cancer than Mexican residents. Liver cancer is a relatively rare cancer type, but its incidence and mortality have been on the rise...

A study suggests working within a religious framework may help eas cultural barriers to cancer screening with mammograms.

A new study showed that one in five young, non-Hispanic white women who tan indoors are dependent on the activity.

A study finds that participation in clinical trials by African-Americans and Hispanics has declined over the last two decades.
Treatment may improve survival for older colorectal cancer patients with dementia, but chemo can be challenging and some may choose to forego care.
The FDA approved a molecularly targeted therapy for treating patients with a certain form of breast cancer.
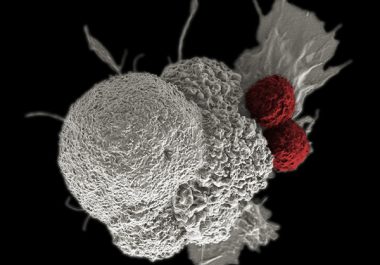
The FDA expanded an immunotherapeutic to used for treating patients with hepatocellular carcinoma – the most common form of primary liver cancer.