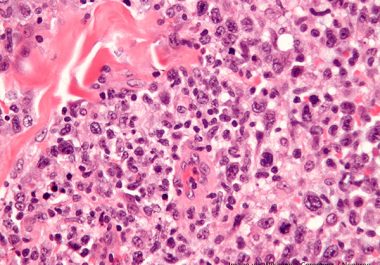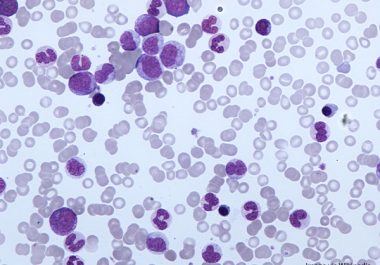
A New Treatment for Chronic Myeloid Leukemia Resistant to Standard Therapies
THE FDA GRANTED ACCELERATED APPROVAL TO A TARGETED THERAPY FOR PATIENTS WITH CML RESISTANT TO OTHER THERAPEUTICS The U.S. Food and Drug Administration (FDA) approved the drug asciminib (Scemblix) for certain patients with Philadelphia...