
Editors’ Picks, July 2024: Risks of CAR T-cell Therapy, Sarcoma Advances, and More
The AACR journal editors' picks for July 2024 include a review of the risks from CAR T-cell therapy, advances made in sarcoma, and more.


The AACR journal editors' picks for July 2024 include a review of the risks from CAR T-cell therapy, advances made in sarcoma, and more.

Learn how liquid biopsy could be used to detect cancer and guide treatment as researchers work to develop more noninvasive screening options.
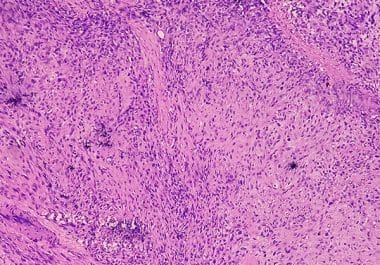
Researchers are making progress on genetic classification of sarcoma tumors, the use of T-cell therapy, and nanomedicine delivery.

Standard screening may not catch endometrial cancer in Black women, and a cancer patient receives a voice box transplant for the first time.

Neuropathy may be prevented with an exercise program, and prostate cancer screening standards may not be appropriate for transgender women taking estrogen.

Immune checkpoint inhibitors are effective against non-small cell lung cancer in some patients. Researchers are investigating who benefits from the drugs and when they should be administered for maximum impact.

With the FDA’s approval of the first tumor‑infiltrating lymphocyte (TIL) therapy to treat advanced melanoma, researchers seek to expand this type of cellular therapy to other solid tumors.

The drug showed promise in treating small cell lung cancer that had progressed during or after chemotherapy.
Acupuncture helps manage side effects of endocrine therapy, and adding chemotherapy before surgery delays progression in pancreatic cancer.

Learn about cancer vaccines, including the various types used to treat or prevent cancer, and discover what's on the horizon.