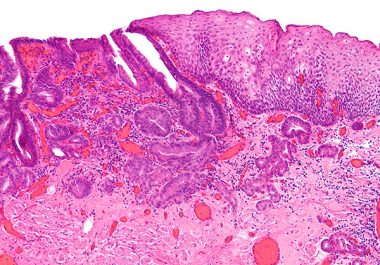
Expanding Immunotherapy to Esophageal Cancer
The FDA approved pembrolizumab, in immunotherapy called a checkpoint inhibitor, to treat certain patients with esophageal cancer.


The FDA approved pembrolizumab, in immunotherapy called a checkpoint inhibitor, to treat certain patients with esophageal cancer.
A new antihormone agent has been approved to treat men with nonmetastatic prostate cancer that has stopped responding to standard antihormone treatments.
A molecularly targeted therapeutic has been approved for treating certain patients with multiple myeloma. Learn more about this recently approved therapy.
An immunotherapeutic known as a checkpoint inhibitor has been approved for the treatment of certain patients with small-cell lung cancer.
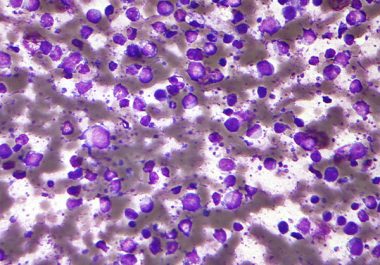
A molecularly targeted therapeutic has been approved to treat some patients with diffuse large B-cell lymphoma the most common type of non-Hodgkin lymphoma.
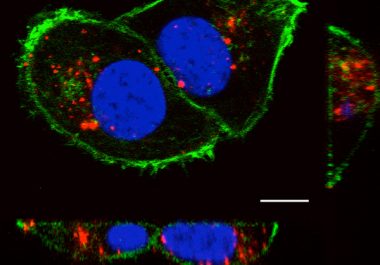
A combination of an immunotherapy and a molecularly targeted therapy has been approved to treat patients with advanced renal cell carcinoma, kidney cancer.
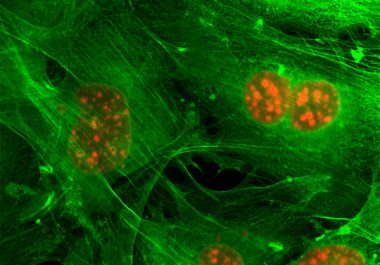
A molecularly targeted therapeutic has been approved for use in combination with fulvestrant for treating certain men and women with breast cancer.
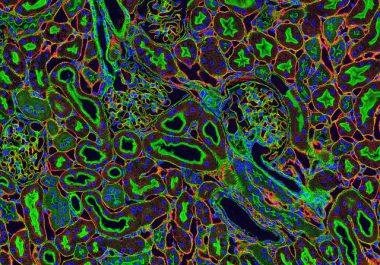
A combination of an immunotherapy and a targeted therapeutic recently was approved by the FDA for patients advanced renal cell carcinoma (kidney cancer).
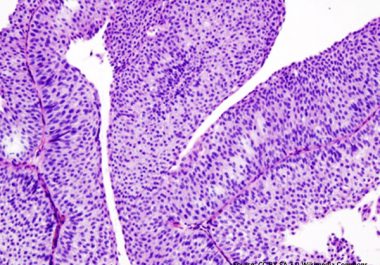
A molecularly targeted therapeutic was approved by the FDA to treating certain patients with urothelial carcinoma, the most common form of bladder cancer.
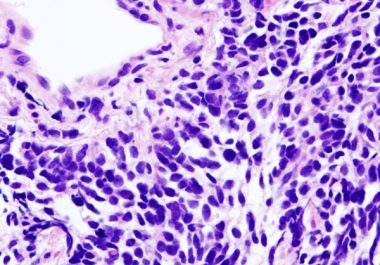
The FDA expanded the use of the immunotherapeutic atezolizumab in combination with carboplatin and etoposide for certain small-cell lung cancer patients.