A New Checkpoint Inhibitor Targeting PD-1
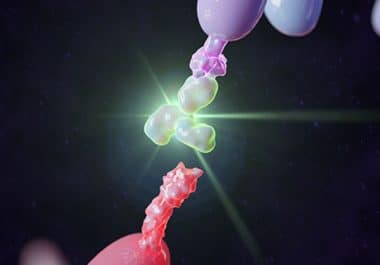
The FDA has approved the monoclonal antibody retifanlimab-dlwr for some patients with Merkel cell carcinoma. The U.S. Food and Drug Administration (FDA) has granted accelerated approval to retifanlimab-dlwr (Zynyz) for the treatment of adult...