New Estrogen Receptor-targeting Therapy for Breast Cancer
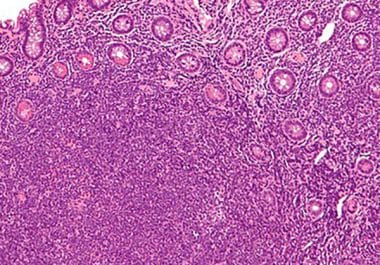
The FDA has approved the oral SERD elacestrant to treat certain patients with estrogen receptor-positive breast cancer. The U.S. Food and Drug Administration (FDA) has approved elacestrant (Orserdu) for the treatment of men and...