March 5: The Week in Cancer News
A patient advocate and researcher argue that the U.S. has set insufficiently ambitious cervical cancer screening goals, and more news of the week from Cancer Today.


A patient advocate and researcher argue that the U.S. has set insufficiently ambitious cervical cancer screening goals, and more news of the week from Cancer Today.
The FDA granted accelerated approval to a CAR T-cell therapy for certain adult patients with follicular lymphoma, a common type of non-Hodgkin lymphoma. The U.S. Food and Drug Administration (FDA) granted accelerated approval to...

Cancer Survivors are more likely than others to walk slowly or have mobility problems that are also linked to higher risk of death, a study found.
Numerous studies have shown that individuals with cancer fare worse than the general population if they contract COVID-19, with higher rates of hospitalization, severe disease, and death. Studies from recent months highlight another new...
The FDA has approved an immune checkpoint inhibitor – a type of cancer immunotherapy – as a first-line therapeutic for certain patients with non-small cell lung cancer.

Nonprofits provide support to young cancer patients in need of fertility preservation, and more news of the week from Cancer Today.
Tool outperformed preexisting mathematical models and a previously established artificial intelligence tool

To learn more about cancer prevention and risk reduction, we talked to Raymond N. DuBois, MD, PhD, FAACR, co-editor in chief of the American Association for Cancer Research (AACR)’s journal Cancer Prevention Research and...
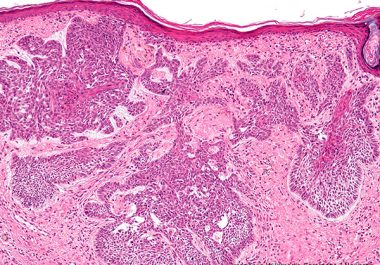
The FDA approved the immune checkpoint inhibitor, cemiplimab-rwlc, for certain patients with locally advanced or metastatic basal cell carcinoma

Organizations call to prioritize cancer patients for COVID-19 vaccination, researchers analyze rates of “low-value” breast surgeries, and UCLA cancer survivors write letters to patients undergoing treatment.