FDA Approves Ovarian Cancer Treatment Based on New Biomarker
On Wednesday, the U.S. Food and Drug Administration (FDA) expanded the use of the molecularly targeted therapeutic niraparib (Zejula) to include an additional group of patients with ovarian cancer, fallopian tube cancer, or primary peritoneal cancer: those whose cancer has progressed despite treatment with at least three different cytotoxic chemotherapy regimens and tests positive for a new biomarker called homologous recombination deficiency (HRD).
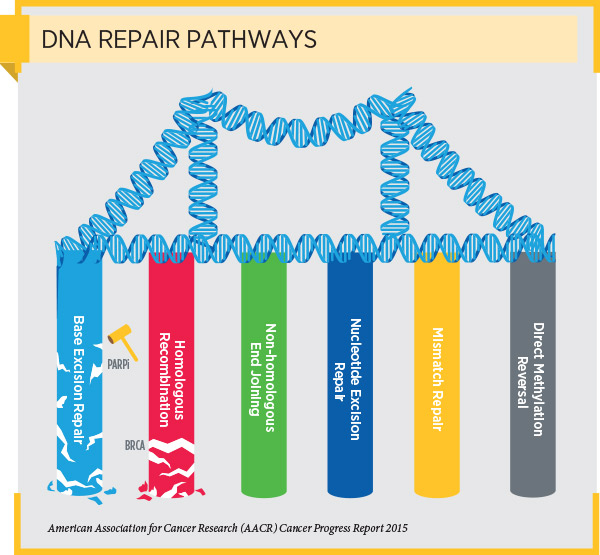
Niraparib targets proteins called poly ADP-ribose polymerase (PARP) proteins, which have a key role in repairing damaged DNA.
DNA damage that causes genetic mutations to accumulate is the primary cause of cancer initiation and progression. As a result, cells have evolved several interrelated pathways that they use to repair damaged DNA and maintain genome stability. One of these pathways is the homologous recombination pathway.
Deficiencies in DNA repair through the homologous recombination pathway lead to the accumulation of DNA damage, a situation in the cell known as genomic instability, which, in turn, can lead to cancer. HRD can arise as a result of loss-of-function mutations in genes encoding proteins involved in the homologous recombination pathway, such as BRCA1, BRCA2, PALB2, ATM, RAD51C, and RAD51D.
As discussed in a previous post on this blog, cells with HRD often repair DNA using an alternative DNA repair pathway called the base excision repair pathway. PARP proteins are important components of the base excision repair pathway. Blocking PARP protein function in cells with HRD prevents the cells from repairing damaged DNA and ultimately causes the cells to die.
This knowledge led to the development of PARP inhibitors. Initially, these molecularly targeted therapeutics were approved by the FDA only for treating patients with advanced ovarian cancer who have an inherited, cancer-associated BRCA1 or BRCA2 (BRCA1/2) mutation. Subsequent approvals expanded the use of some of these therapeutics to include the treatment of patients with metastatic or locally advanced, HER2-negative breast cancer who have an inherited, cancer-associated BRCA1/2 mutation and the maintenance treatment of patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancers, irrespective of BRCA mutation status, that are responding to platinum-based chemotherapy.
The first FDA approval for niraparib was announced in March 2017. It was for use of the molecularly targeted therapeutic as a maintenance treatment for adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancers that are responding to platinum-based chemotherapy. It was the first approval of a PARP inhibitor for use in treating both BRCA mutation–positive and –negative cancers.
According to the FDA statement, the new approval of niraparib for treating patients with advanced ovarian, fallopian tube, or primary peritoneal cancer that tests positive for HRD was based on results from the phase II QUADRA clinical trial. The results showed that 24 percent of 98 patients who had HRD-positive advanced ovarian cancer had a partial response. These responses lasted an estimated median of 8.3 months.
Alongside the niraparib approval, the FDA approved a companion diagnostic to identify patients eligible for the new treatment: those who have HRD-positive advanced ovarian, fallopian tube, or primary peritoneal cancer. The companion diagnostic, which is called myChoice CDx, determines HRD status by testing for the presence of cancer-associated BRCA1/2 mutations and three other markers of genomic instability: loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions.
There are many clinical trials underway investigating the use of niraparib and other PARP inhibitors as a treatment for other cancers testing positive for HRD, including prostate cancer. We look forward to providing updates on this blog of any future advances in the use of the HRD biomarker in selecting treatments for cancer patients.