
Let Me Tell You a Story About the Power of Medical Research
Ten-year-old Michael Methner told his story about his experience being diagnosed with optic nerve glioma at the Rally for...


Ten-year-old Michael Methner told his story about his experience being diagnosed with optic nerve glioma at the Rally for...

This quarter’s 16 approvals include a first-in-class cell therapeutic, a flurry of new options for EGFR-mutated lung cancer, and...

Expanding clinical trial eligibility requirements can help increase participation and more accurately show how treatments work in a real-world...
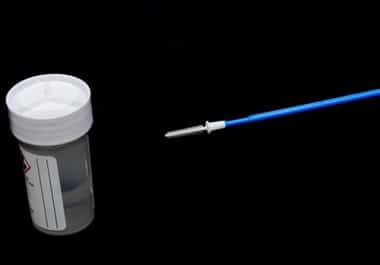
New cervical cancer self-screening options and a portable blood test device could lead to changes in at-home cancer screening...

Highlights from a workshop and journal article that shared key considerations for including overall survival as an endpoint in...

The FDA issued 18 oncology-related approvals in Q2 2024 including two first-in-class drugs and a bispecific T-cell engager for...
Dr. Edward Cliff presented a poster on the clinical benefit of cancer drugs granted accelerated approval.

The FDA issued 14 oncology approvals this quarter, including the first tumor-infiltrating lymphocyte therapy.

In the final quarter of 2023, the FDA issued 17 approvals specifically for the treatment of tumors.
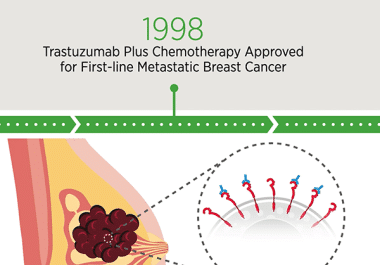
The AACR Annual Meeting 2023, held April 14-19, commemorated several milestones, including the 25th anniversary of the approval of...