FDA Approvals in Oncology: October-December 2023
With the approval of new anticancer therapeutics, more treatment options become available for patients. Some therapies are new to the market, while some may have already been approved for other indications; some molecules are first in class, directed against a previously untargeted pathway or acting through a new mechanism, while others may be improved versions of drugs that already exist.
To help our readers keep track of the cancer therapies approved by the U.S. Food and Drug Administration (FDA), understand their impact for patients, and put them in context of the current therapeutic landscape, Cancer Research Catalyst provides a quarterly review of the latest approvals from the FDA.
The final three months of 2023 were chock-full of FDA drug approvals, with an incredible 17 approvals specifically for the treatment of tumors.
These approvals closed out a busy year for the FDA that featured 45 oncology drug approvals, including 17 for drugs new to the market. Approvals from January through September were discussed in previous blog posts:
- FDA Approvals in Oncology: January-March 2023
- FDA Approvals in Oncology: April-June 2023
- FDA Approvals in Oncology: July-September 2023
Here, we will provide an overview of the approvals from the final quarter of 2023, which included new indications for immune checkpoint inhibitors and various targeted therapies.
New immunotherapy options
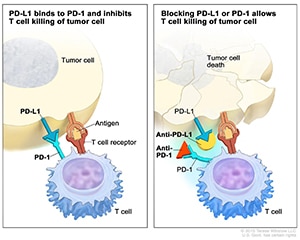
The immune system can detect and eliminate dangerous components, including cancer cells, from the body. To prevent overactivation of the immune system, some immune cells are equipped with an ‘off’ switch, PD-1, that turns off immune signaling upon binding to its partner, PD-L1. High expression of PD-L1 on some cancers keeps the regulatory switch in the ‘off’ position, allowing cancer cells to escape antitumor immunity.
Immune checkpoint inhibitors are a type of immunotherapy that prevents the protein PD-L1 from engaging the protein PD-1 on immune cells, thereby allowing immune signaling to turn ‘on.’ Immune checkpoint inhibitors were initially approved for progressive or recurrent disease, but studies in recent years have shown that these drugs may be effective in earlier lines of treatment as well.
This quarter featured five new approvals for immune checkpoint inhibitors, including several for first-line therapy.
- Toripalimab (Loqtorzi) was approved in combination with the chemotherapies gemcitabine (Gemzar) and cisplatin for the first-line treatment of patients with metastatic, locally advanced, or recurrent nasopharyngeal carcinoma. Toripalimab was also approved as a single agent for nasopharyngeal carcinoma that did not respond to or relapsed following treatment with platinum-based chemotherapy.
These are the first FDA approvals for toripalimab, a PD-1 inhibitor that has a 12-fold higher binding affinity to PD-1 compared to pembrolizumab (Keytruda), according to research presented at the 2023 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. This could allow for improved immune checkpoint blocking.
- Pembrolizumab (Keytruda) was approved for three new indications:
- As first-line therapy in combination with platinum chemotherapy before surgery and as monotherapy after surgery in patients with surgically removable non-small cell lung cancer (NSCLC). This approval expands an earlier approval from 2023 that made single-agent pembrolizumab available for postsurgical use in patients with resectable NSCLC.
- In combination with gemcitabine and cisplatin for the treatment of locally advanced unresectable or metastatic biliary tract cancer, including in the first-line setting.
- In combination with a chemotherapy regimen for the first-line treatment of locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma that does not overexpress HER2. Pembrolizumab was previously approved alongside HER2-targeted therapy and chemotherapy for HER2-positive gastric or GEJ adenocarcinomas. This approval expands pembrolizumab’s use to HER2-negative tumors.
- In addition, an earlier accelerated approval of a regimen containing pembrolizumab and enfortumab vedotin (Padcev) for certain bladder cancers was converted to a full approval this quarter.
- Nivolumab (Opdivo) was approved for the postsurgical treatment of stage 2B/C melanoma that has been completely resected, including for patients as young as 12 years old. Nivolumab was previously approved for unresectable or advanced melanoma; the approval from this quarter allows it to be used for locoregional disease, including in the first-line setting.
Inhibitors of receptor tyrosine kinase signaling
Receptor tyrosine kinases (RTKs) activate signaling pathways that promote cellular proliferation. These pathways are frequently dysregulated in cancer cells, leading to unfettered cancer cell expansion. To slow the growth of cancer, tyrosine kinase inhibitors (TKIs) may be employed against some of the most commonly mutated proteins in cancer, such as those in the MAPK and PI3K signaling pathways.
This quarter, several new TKI regimens were approved.
- Encorafenib (Braftovi) combined with binimetinib (Mektovi) was approved for metastatic non-small cell lung cancers (NSCLC) with the BRAF V600E mutation, including in the first-line setting. Encorafenib and binimetinib inhibit BRAF and MEK, respectively, both of which are proteins within the MAPK signaling pathway. Simultaneous inhibition of BRAF and MEK reduces the risk of treatment resistance.
- Repotrectinib (Augtyro) was approved to treat locally advanced or metastatic NSCLC that harbors a genetic alteration involving the ROS1 gene. It is designed to inhibit mutated forms of three RTKs that can drive tumor growth—ROS1, NTRK, and ALK—and can block the activity of mutations that confer resistance to other ROS1, NTRK, or ALK inhibitors. Repotrectinib is the first ROS1 inhibitor that has been approved to treat patients whose tumors did not respond to or relapsed following treatment with a previous ROS1 inhibitor.
- Capivasertib (Truqap) was approved in combination with the endocrine therapy fulvestrant (Faslodex) to treat progressive or recurrent locally advanced or metastatic hormone receptor (HR)-positive, HER2-negative breast cancer that harbors one or more mutations in PIK3CA, AKT1, or PTEN. Mutations in these genes are common in HR-positive, HER2-negative breast cancers and may lead to resistance to endocrine therapy. Inhibiting the proteins encoded by these mutant genes may, therefore, help overcome resistance.
This is the first FDA approval for capivasertib, which works by inhibiting the activity of AKT, a protein within the PI3K signaling pathway. Since PTEN and PIK3CA are also in this pathway, inhibiting AKT may block the activity of these proteins as well. Results from the clinical trial that led to the approval were presented at the 2022 San Antonio Breast Cancer Symposium, which the AACR cosponsors.
- Fruquintinib (Fruzaqla) was approved for adult patients with metastatic colorectal cancer (mCRC) who have received certain prior therapies. Fruquintinib binds to a family of RTKs called VEGF receptors and prevents them from sending signals that promote tumor cell proliferation. VEGF receptor blockade also hinders the growth of new blood vessels that bring essential oxygen and nutrients to the tumor. This is the first FDA approval for fruquintinib.
- Entrectinib (Rozlytrek) was approved for certain pediatric patients older than 1 month who have metastatic or unresectable solid tumors that harbor a gene fusion involving the NTRK gene, and who have progressed on prior treatment or have no other satisfactory treatment options. Entrectinib was previously approved for patients aged 12 and older. The current approval allows patients as young as 1 month to receive treatment. Entrectinib inhibits the activity of NTRK, an RTK that can drive tumor growth when fused with part of another gene.
- Pirtobrutinib (Jaypirca) was approved for chronic lymphocytic leukemias (CLL) or small lymphocytic lymphomas (SLL) that have received at least two prior lines of therapy, including another inhibitor of Bruton’s tyrosine kinase (BTK). Pirtobrutinib blocks the activity of BTK, which is commonly expressed on the B cells that make up certain leukemias and lymphomas.
Because pirtobrutinib binds to BTK in a different way than other BTK inhibitors, it can sometimes benefit patients whose cancers have become resistant to other BTK inhibitors. Pirtobrutinib was previously approved for mantle cell lymphomas that had progressed after treatment with another BTK inhibitor.
Additional drug approvals
In addition to the immune checkpoint inhibitors and TKIs discussed above, several other drugs were approved or received new indications this quarter:
- Enzalutamide (Xtandi), a hormone therapy, received a new indication for certain high-risk and recurrent castration-sensitive prostate cancers.
- Ivosidenib (Tibsovo), which inhibits mutated forms of the metabolic enzyme IDH1, received a new indication for relapsed or treatment-refractory myelodysplastic syndromes with IDH1 mutations.
- Belzutifan (Welireg), an inhibitor of the hypoxia-responsive HIF2α transcription factor, received a new indication for advanced kidney cancers that received prior immunotherapy and a VEGF TKI.
- Elfornithine (Iwilfin), an inhibitor of an enzyme involved in RNA and DNA synthesis, received its first FDA approval for cancer—to reduce the risk of recurrence in adult and pediatric patients with high-risk neuroblastoma that responded to a prior treatment regimen containing anti-GD2 immunotherapy. Elfornithine is the first FDA-approved treatment for reducing the risk of relapse in pediatric patients with this disease.
- Nirogacestat (Ogsiveo), which suppresses Notch signaling, was approved for painful noncancerous growths called desmoid tumors. This is the first FDA approval for nirogacestat and the first FDA-approved treatment for desmoid tumors.


