Investigating New Approaches to Treat Metastatic Kidney Cancer
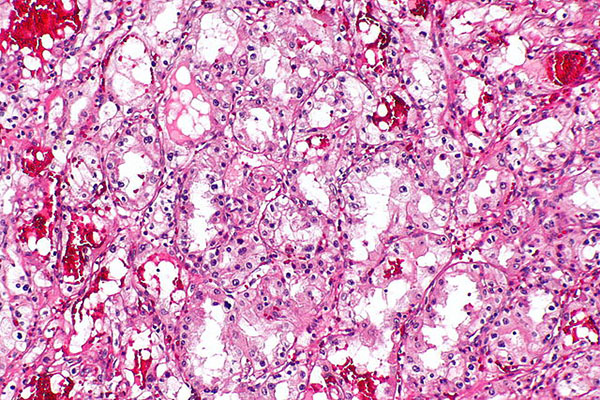
Kidney cancer is the eighth most commonly diagnosed cancer in the United States, with a five-year survival rate of 72.4 percent. At first glance, these survival rates compare advantageously to other seemingly deadlier cancers such as those of the pancreas or lung, which have five-year survival rates of 6.7 percent and 16.8 percent, respectively. However, it is important to note that while the five-year survival of localized kidney cancer is very high (91.8 percent), that of metastatic kidney cancer is only 12.1 percent. These statistics emphasize the need to better understand what drives the progression of kidney cancer and to develop more effective treatments for patients with advanced disease.
One of the researchers leading this charge is Scott M. Welford, PhD, assistant professor of radiation oncology at Case Western Reserve University School of Medicine. Welford, the recipient of the 2014 Kure It-AACR Grant for Kidney Cancer Research, heads a research program investigating the impact of hypoxia (low levels of oxygen) on tumor initiation and resistance to therapy in kidney cancer and glioblastoma.

Dr. Welford receiving his award. With him are, left to right, Barry L. Hoeven, founder, Kure It Cancer Research; Todd Perry, board member, Kure It Cancer Research; and Charles L. Sawyers, MD, immediate past president of the AACR.
His interest in kidney cancer, specifically clear cell renal cell carcinoma (ccRCC), stems from its unique molecular etiology. “Kidney cancers are unusual in that the hallmark genetic lesion is mutation or alteration in the von Hippel Lindau gene product, whose normal role is to control the hypoxia inducible factors (HIFs), which are transcription factors responsible for regulating hypoxia,” Welford remarks. “So kidney tumors think and act like they are hypoxic all the time, creating a nice model system to study hypoxic responses than can be applicable to a vast number of solid tumors.”
Tumor hypoxia is remarkably common, and is observed across a wide range of cancer types. The development of hypoxia within a tumor often is simply a physical manifestation of the tumor outgrowing its blood supply. “As a result,” Welford says, “tumor cells activate stress response signals to increase vascularity and change metabolism, predominantly via the activation of the HIFs.”
Welford’s long-standing interest in hypoxia and his solid foundation in molecular biology and biochemistry inform the approaches he takes to interrogate the tumor microenvironment. “Hypoxia impacts tumor development, aggressiveness, and chemotherapeutic and radiation responses,” he says. “I am fascinated by the coercion of a stress response pathway that has shaped not only our evolution, but also our individual development, into a major complicating factor in tumor therapy.”
His Kure It-AACR funded project encompasses preclinical studies that seek to define new molecular targets that are regulated by hypoxia in ccRCC, with the long-term goal of developing a new therapeutic that could complement the current standard of care for ccRCC patients. Welford and his team are particularly focused on two specific HIF targets commonly expressed in ccRCC, the macrophage migration inhibitory factor (MIF) and D-dopachrome tautomerase (DDT). His group previously showed that reducing the expression of these targets has dramatic effects on tumorigenic capacity of ccRCC cells; however, there are currently no effective mechanisms to simultaneously target both proteins. His project is designed to overcome this limitation, by designing a new soluble receptor that can serve as a dual inhibitor of NIF and DDT.
“Our project investigates what we hope will be a novel therapeutic approach in kidney cancer based on our identification of a family of cytokines that promote tumor cell survival, endothelial cell migration, and inflammatory cell infiltration,” Welford says. “We hypothesize that by eliminating the function of these cytokines, renal carcinoma cells will become more susceptible to chemotherapies and radiation treatment, leading to synergistic responses to existing treatments.” Only six months into the two-year funding term, Welford and his team are off and running on the project, making important strides toward accomplishing their research objectives.
As he continues this important work, Welford acknowledges that the impact of the funding provided by this Kure It-AACR grant has been tremendous. “In the current NIH funding landscape, the mission of funding high-risk/high-reward projects by foundation grant opportunities allows young investigators like me to take risks toward achieving real and palpable benefits to cancers where treatment options are limited.”