FDA Approves New Treatment for Certain Patients With Leukemia
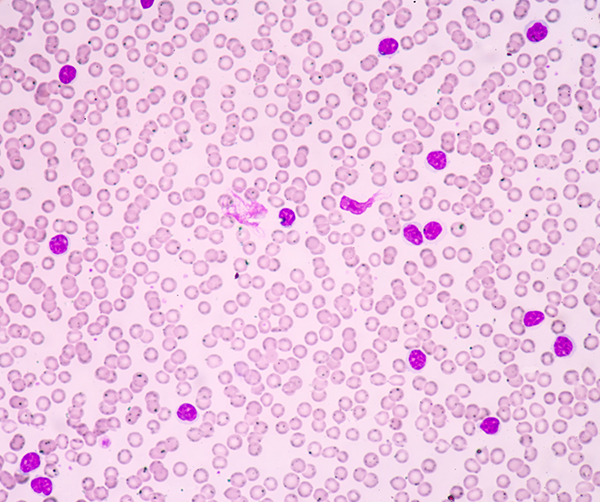
Last week, the U.S. Food and Drug Administration (FDA) added another therapeutic to the armamentarium for hematologist-oncologists when it approved venetoclax (Venclexta) for the treatment of certain patients with chronic lymphocytic leukemia (CLL). Specifically, the new anticancer therapeutic was approved for patients who have CLL characterized by a genetic abnormality called the 17p deletion and whose disease has previously been treated with at least one other therapeutic.
CLL is the second most common form of leukemia diagnosed in the United States, with almost 19,000 new cases anticipated in 2016. According to the FDA in its announcement about venetoclax, the 17p deletion occurs in about 10 percent of patients with untreated CLL and about 20 percent of patients whose disease has relapsed after initially responding to treatment. Patients with this genetic abnormality tend to have worse outcomes than those without it.
Venetoclax is the first in a new class of anticancer therapeutics called BCL-2 inhibitors. BCL-2 is a protein that promotes cell survival by preventing cells from undergoing a natural self-destruct process called apoptosis. CLL cells often express elevated levels of BCL-2, and by blocking this protein, venetoclax triggers the cells to die by apoptosis.
The FDA decision about venetoclax was based on the initial results from a phase II clinical trial that showed that 79.4 percent of patients responded to treatment. For most of these patients, the response was still ongoing at 12 months.
The treatment landscape for patients with CLL has changed markedly over the past three years, with the approval of obinutuzumab (Gazyva), ibrutinib (Imbruvica), idelalsib (Zydelig), and now venetoclax. Each of these therapeutics exerts its antileukemia effects in a different way, highlighting how our increasing knowledge of the biology of CLL has identified multiple different therapeutic targets. It is advances like these that fill David Rampe, a patient with CLL who was featured in the AACR Cancer Progress Report 2014, with confidence for the future. “Even if my disease relapses at some point, I am confident that other new drugs will be available to help manage my condition,” he said.
Find out more about David’s story in this video: