NIH-AACR Cancer, Autoimmunity, and Immunology Conference: Virtual Presentations Highlight Advances in Managing Adverse Events
“These are extraordinary times,” began Hugh Auchincloss, MD, as he opened up the recent NIH-AACR Cancer, Autoimmunity, and Immunology Conference, a meeting that was conducted entirely online due to the COVID-19 pandemic.
Auchincloss, principal deputy director of the National Institute of Allergy and Infectious Diseases (NIAID), set the stage for this conference, which largely revolved around immune-related adverse events (irAEs) associated with immunotherapy regimens.
As more patients receive immunotherapy, the associated side effects have become an important focus in the field. Any organ can be subject to inflammation driven by hyperactivation of the immune system. These irAEs are distinct from side effects from more traditional therapies, and as the field of immunotherapy continues to evolve, knowledge about the underlying mechanisms of irAEs – along with the best way to manage them without impeding anticancer effects – continues to grow.
The conference, which was held March 23-24, was a virtual meeting place for the communication of the latest research about irAEs, which incorporated interactive panel discussions that integrated questions from the online audience. While presenters and attendees were “socially distant,” the meeting sparked real-time conversations among scientists and patients about this key area of study.
Seeing is believing: using MRI for early evaluation of rheumatologic iraes
The first session of the meeting focused on rheumatologic toxicities associated with immune checkpoint inhibitors. Talks in this session highlighted the differences between classic versus immunotherapy-induced rheumatologic diseases, along with a discussion on the severity of myositis associated with immune checkpoint blockade. The final talk in this session, delivered by Sarthak Gupta, MD, from the National Institute of Arthritis and Musculoskeletal and Skin Diseases in Bethesda, Maryland, reviewed a recent study that used magnetic resonance imaging (MRI) to assist in the diagnosis of inflammatory arthritis induced by treatment with immune checkpoint inhibitors.
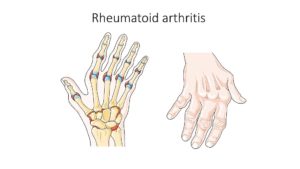
Inflammatory arthritis, which is characterized by painful joint stiffness and swelling, can also cause bone erosion that may never heal, resulting in lifelong challenges. While it is estimated that around 40 percent of patients receiving immune checkpoint inhibitors in clinical trials have arthralgia (joint pain), a single-institution report found that around 2 percent of patients receiving this treatment were clinically diagnosed with inflammatory arthritis, noted Gupta. Importantly, inflammatory arthritis can persist after cessation of treatment, resulting in decreased quality of life for the patient.
X-rays, which are often used as part of clinical evaluation, are not sensitive enough to detect early bone damage and degenerative changes, Gupta said. While ultrasound has been used for this purpose, a major limitation of this approach is inter-operator variability. As such, a sensitive and reproducible method to detect patients at high risk for joint damage is needed to study those with checkpoint inhibitor-induced arthralgia.
In a retrospective case series, Gupta and others utilized MRI to assess eight patients that developed musculoskeletal issues upon receiving immune checkpoint inhibitors. At least one joint from each patient was evaluated, resulting in an overall assessment of 13 joints. Tenosynovitis (inflammation of the tendon) and synovitis (inflammation of the lining of synovial joints), both of which can cause lasting damage if left unchecked, were frequently detected by MRI, even in patients with minimal symptoms. Further, MRI identified three patients with bone marrow edema and erosions, which are early indications of bone damage. These results suggest that bone damage may be common and underreported in this patient population.
Gupta noted that MRI has the potential to help clinicians identify patients at higher risk of erosive disease, and to accurately grade irAEs and make treatment decisions, such as when to resume checkpoint blockade therapy if treatment was paused.
The microbiome and myocarditis: treating the gut to heal the heart
Attention in the cancer field has recently focused on the gut microbiome, and research detailing how the composition and modulation of this diverse ecosystem may affect survival outcomes has come to the fore. One session in the conference hosted three presentations on recent microbiome research, and the final talk, delivered by Burkhard Ludewing, DVM, from Kantonsspital St. Gallen in Switzerland, centered on the link between the gut microbiome and myocarditis.
Myocarditis, or inflammation of the heart muscle, is a rare irAE that has been linked to the use of checkpoint inhibitors. Despite its low incidence, the mortality rate of this complication has been reported to be as high as 50 percent.
To determine whether the gut microbiome can affect myocarditis, Ludewing and colleagues evaluated a mouse model of spontaneous myocarditis housed under two conditions: specific-pathogen-free (SPF) conditions, where the animals are kept in an environment that lacks certain microorganisms, compared with germ-free (GF) conditions, where the mice are kept in an environment free of all microorganisms (notably those that make up the gut microbiome).
All animals developed spontaneous autoimmune myocarditis. While approximately 50 percent of SPF mice exhibited lethal cardiomyopathy at 20 weeks of age, all of the GF mice, which lack a commensal gut microbiome, survived during this time frame. Further, GF mice had reduced cardiac dilatation, cardiac fibrosis, and disease severity. If GF mice were recolonized with the microbiome from SPF mice, the animals exhibited exacerbated cardiac inflammation, impaired cardiac function, and progression to lethal disease. Modulation of the gut microbiome with antibiotic treatment, which reduced the abundance of B. theta, a common anaerobic bacterium found in the human gut, decreased the disease severity and increased the survival of mice with myocarditis.
Analysis of serum from patients with acute myocarditis revealed significantly elevated B. theta-specific immunoglobulin G (IgG) responses when compared with healthy controls. Additionally, as patients improved clinically, their seroreactivity against B. theta decreased accordingly.
These results suggest that antibiotic treatment may provide an efficacious strategy to combat myocarditis, a potentially fatal irAE.
Pembrolizumab for PML: targeting an immune checkpoint to counteract brain infection
One session of the meeting discussed the interplay between infectious diseases and the immune system, and presentations demonstrated how the disruption of immune checkpoints could increase the risk of bacterial infections, such as tuberculosis, or could be used as a treatment modality for viral infections, such as the John Cunningham virus (JC virus).
The JC virus is a common polyomavirus that is usually harmless in humans. However, in immunocompromised individuals, including patients with cancer, infection with the JC virus can lead to progressive multifocal leukoencephalopathy (PML), a devastating brain infection with a high mortality rate. Further, survivors often have lifelong neurological complications.
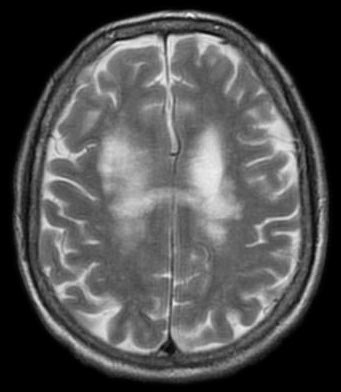
While the incidence of PML is rare, treatment-related PML is on the rise, noted Irene Cortese, MD, from NIAID, in the final presentation of this session. In fact, there are 17 drugs approved by the U.S Food and Drug Administration (FDA), including several immunosuppressants, that carry a warning for PML, she said.
There are no validated antiviral therapies for PML, and the only effective treatment for the disease is immune reconstitution. This treatment, however, has a life-threatening complication: an overactive immune response known as immune reconstitution inflammatory syndrome (IRIS). As such, new treatment approaches are needed for this potentially lethal viral infection.
Previous work has shown that the expression of the immune checkpoint PD-1 was enriched on JC virus-specific T cells, and that the in vitro blockade of PD-1 augmented antiviral activity in a subset of patients. To build upon this work, a study, recently published in The New England Journal of Medicine by Cortese and others, evaluated the PD-1 inhibitor pembrolizumab (Keytruda) in eight patients with PML with a variety of underlying diseases.
The researchers found that the drug was safe and well-tolerated, and no patients had treatment-limiting toxicity or symptomatic IRIS. In all eight patients, treatment with pembrolizumab resulted in reduced PD-1 expression on CD4 and CD8 T cells in both the blood and cerebral spinal fluid (CSF). Further, five patients had clinical and radiological improvement or stabilization of disease with accompanying decreases in JC viral load in the CSF. Of these patients, three were still alive at more than two years after the last infusion, Cortese noted. The remaining two patients died more than a year after the last infusion due to underlying cancer, she said.
Cortese concluded the session by noting that this proof-of-principle study demonstrates that additional investigation of checkpoint inhibitors for the treatment of PML is warranted.
Programmable bacteria: using E. coli to deliver an immunotherapeutic payload
Another session of the conference highlighted the use of nontraditional nonclinical models, spanning from woodchucks to pet canines, to test immunotherapies. One organism, however, was not used to test treatment strategies, but was rather a treatment strategy in itself – a bacterium engineered to lyse, or break down, in the tumor microenvironment and release a therapeutic payload.
Bacteria selectively grow in tumors, said Tal Danino, PhD, from Columbia University in New York, as he began his talk. The environment of the tumor – which is hypoxic, has a low pH, and is laden with necrotic tissue – is amenable to bacterial growth, he said. Further, because immune surveillance is low within the tumor core, this provides a “safe haven” for the bacteria to colonize. This unique phenomenon enables for tumor targeting independent of tumor genetics, he added.
In a study recently published in Nature Medicine, Danino and others created a bacterial cancer therapy using a non-pathogenic E. coli strain which contains a synchronized lysis circuit (SLC). The SLC facilitates intratumoral quorum-lysis, or the simultaneous lysis of bacterial cells once the colony reaches a certain size, enabling the coordinated delivery of the therapeutic drug within the tumor. The researchers engineered this E. coli strain to release a nanobody (a single-domain antibody) against CD47, a protective receptor that is highly expressed on certain tumor cells and inhibits phagocytosis. In addition to increased phagocytosis, research has shown that inhibition of CD47 promotes cross-presentation of tumor antigens, facilitating T-cell mediated antitumor activity.
The researchers tested the genetically modified E. coli in mice with subcutaneous lymphoma by injecting the bacteria directly into the tumors. Bacterial treatment resulted in durable tumor regression and prevention of metastasis in the mice. Further, local injection with the bacteria reduced the growth of untreated tumors, suggesting the abscopal effect of this approach.
This “bacterial immunotherapy” can be tailored as needed to deliver other nanobodies or cytokines, allowing for targeted delivery of immunotherapeutics that can be toxic when given systemically.
Coming together: a national biorepository for patients with iraes, hyperprogression, and rare infections will include a COVID-19 arm

Despite the COVID-19 pandemic, “science will go on, and the NCI functions will still go on,” said Norman “Ned” Sharpless, MD, FAACR, director of the National Cancer Institute (NCI), during the final day of the conference. One such NCI initiative is the establishment of a national biorepository of samples from patients who experience irAEs, with the goal of better understanding how to predict, prevent, and treat these side effects. This initiative, along with several other multidisciplinary collaborative efforts, was the focus of the final session of the conference.
The national biorepository initiative – named Alliance A151804 – is led by David Kozono, MD, PhD, from the Dana-Farber Cancer Institute in Boston, who gave an overview of the biobank at the conference. This collaborative effort, activated by the newly formed Alliance Immuno-Oncology Committee, will allow for the systematic study of samples taken from patients receiving immunotherapy. Within 72 hours of a confirmed irAE of grade 3 or higher, patients will be eligible to register for this trial. Then, within a week of registration, the patient will undergo collection of tissue, blood, and optional stool samples. Samples will also be collected one month after registration, and sample collection will continue until irAE resolution or until one year following the irAE event.
Kozono noted that biospecimen collections are traditionally timed to the treatment and assessment of tumor responses, rather than the onset of irAEs. This biobank will allow for the standardized prospective collection of patient samples to specifically address irAEs.
The biorepository will also collect biospecimens from patients that develop hyperprogression or develop a rare infection (for example, a fungal or mycobacterial infection) following initiation of immunotherapy. Kozono mentioned that this rare infection cohort will now include patients with COVID-19.
This initiative was centrally activated on January 31, and 169 sites were activated as of February 29. The first subject was enrolled in this trial on March 12.
“It’s very important that science continues in this challenging time,” Sharpless said, referring to the COVID-19 pandemic. Virtual meetings, like this one, facilitate the continued sharing of key research data and allow for science to keep moving forward.



