Advances in Malignant Lymphoma Virtual Meeting: Understanding and Improving Responses to Immunotherapy
Lymphomas are a form of blood cancer that begin in lymphocytes, also known as white blood cells. Over the past decade, immunotherapy, a form of cancer treatment that uses a patient’s immune response to recognize and destroy cancer cells, has dramatically altered the treatment landscape for lymphomas, as well as for many other cancer types.
There are several types of immunotherapy, each of which uses a different strategy to stimulate an antitumor immune response. Chimeric antigen receptor T-cell (CAR-T) therapy is a form of adoptive cell therapy in which a patient’s T cells are extracted, engineered, multiplied, and reintroduced into the patient. The engineered T cells express a chimeric antigen receptor (CAR) that binds to antigens on the surface of cancer cells and activates immune signaling within the T cell via the CAR’s signaling domains. CAR-T therapy has been highly successful in treating some patients with blood cancers, and three CAR-T therapies are currently approved for the treatment of certain lymphomas in the United States.
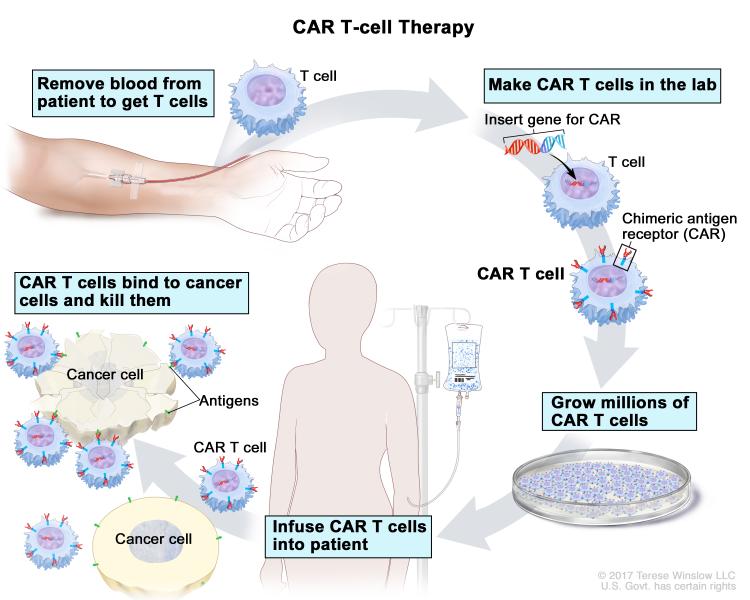
Other types of immunotherapy include immune checkpoint inhibitors, some of which are monoclonal antibodies that bind to proteins on either cancer cells or immune cells to relieve immune suppression; and other monoclonal antibodies that bind to tumor antigens to stimulate an antitumor immune response.
Despite the successes of these therapies in some patients with lymphoma, immunotherapy is not effective for the majority of patients, and resistance can develop in patients who initially respond. Additional research is needed to better understand mechanisms of resistance and to improve responses to immunotherapy.
In honor of Blood Cancer Awareness Month, we will describe some of the latest immunotherapy research presented at the AACR Virtual Meeting: Advances in Malignant Lymphoma, held online August 17-19. Presentations at the meeting described some of the factors and mechanisms underlying nonresponsiveness to immunotherapy in lymphoma and highlighted potential strategies for improving immunotherapy for this blood cancer.
Why is immunotherapy ineffective for some lymphomas?
As explained in a presentation by Stephen Ansell, MD, PhD, professor of medicine at the Mayo Clinic, the tumor microenvironment of lymphoma contains a complex mixture of cells that can contribute to immune cell exhaustion and suppression, thereby hindering the response to immunotherapy in some patients. Certain components of the tumor microenvironment target malignant cells for destruction, while others are recruited by the lymphoma to promote tumor growth.
“It is critical for us to understand this complexity and then specifically look at ways in which we might circumvent the immune suppression or exhaustion [that] exists in lymphoma,” said Ansell.
In his presentation, Ansell highlighted studies from the past two decades that have contributed to our understanding of immune suppression and exhaustion in lymphoma. He explained that immune suppression may occur due to cells in the tumor microenvironment that directly suppress antitumor T cells. These suppressive cells include regulatory T cells, macrophages, and myeloid-derived suppressor cells. In addition, certain subsets of regulatory T cells, such as those that express the immune checkpoint protein TIGIT, may be more immunosuppressive than other subsets. The cytokine IL-10 also contributes by promoting the expansion of immunosuppressive monocytes.
Ansell also reviewed the factors that contribute to T-cell exhaustion, which is observed in a “sizeable proportion” of the T cells from patients with follicular lymphoma. He explained that immune-stimulating cytokines can paradoxically dampen the immune response by inducing T-cell exhaustion due to persistent immune activation. This leads to inadequate cytokine production and reduced T-cell proliferation. Furthermore, studies have shown that T cells that express PD-1 and TIM-3 are less proliferative than cells without these proteins.
Ansell concluded by stating, “While cells might be exhausted, while they may be suppressed, there is a significant opportunity using available agents to be able to change their function and thereby improve the outcome of lymphoma patients therapeutically.” Potential strategies for preventing T-cell exhaustion and immune suppression include inhibition of TIM-3 or PD-1, activation of T cells by signaling through the CD-27 stimulatory receptor, and/or inhibition of regulatory T-cell function.
In addition to contributions from the tumor microenvironment, various tumor, patient, and treatment characteristics may hinder the efficacy of immunotherapy for patients with lymphoma, according to another presentation by Sattva Neelapu, MD, a professor of lymphoma and myeloma at MD Anderson Cancer Center. He proposed that tumor characteristics that could impact the response to CAR-T therapy include tumor burden or subtype, tumor antigen density, loss of the tumor antigen, or antigen escape (when the immune system no longer responds to the tumor antigen). Furthermore, characteristics of the patient, such as age, performance status, comorbidities, and prior treatments, could affect the response. Additionally, the tumor’s response may be impacted by the dose, signaling domains, or structural components of the CAR T cell.
Improving immunotherapy for lymphoma
Studies presented at the meeting also examined potential new strategies for improving immunotherapy for lymphoma. An existing immunotherapeutic approach is to use monoclonal antibodies to stimulate an immune response against cancer cells; however, the resulting immune stimulation can vary, and the reasons for the varying levels of stimulation are unclear. Mark Cragg, PhD, professor of experimental cancer research at University of Southampton, investigated potential factors that could influence the strength of immune stimulation in response to monoclonal antibodies. Cragg and colleagues studied antibodies targeting the CD40 receptor, which is found on cell surfaces and is a potent stimulator of the immune system. They found that mouse CD40-targeted antibodies triggered clustering of CD40 receptors, which was required for downstream signaling and the release of inflammatory cytokines. In addition, they determined that the isotype of the antibody and the epitope that it targeted impacted how effectively the antibody was able to stimulate an immune response.
The researchers then examined human CD40-targeted antibodies and converted an antibody that inhibited downstream immune signaling to one that stimulated immune signaling by changing its isotype. Using a mouse model, Cragg and colleagues showed that administration of the engineered CD40 antibody decreased tumor volume and increased survival. Together, the results showed that CD40 receptor clustering is required for downstream immune-stimulating signaling and that the epitope and isotype can affect the immune-stimulating activity of monoclonal antibodies. These findings could eventually contribute to the development of improved therapies for lymphoma.
In another presentation, Renier Brentjens, MD, PhD, one of the session moderators and director of cellular therapeutics at Memorial Sloan Kettering Cancer Center, discussed strategies for improving CAR T-cell therapy. He explained that CAR T cells that secrete the IL-18 cytokine, express the CD40 ligand, or secrete a PD-1-blocking single-chain variable fragment have been shown to improve survival in mouse models.
Brentjens also described a recently published study from his group that investigated the efficacy of CAR T cells secreting the IL-36γ cytokine, a potent stimulator of the adaptive immune response. Brentjens and colleagues found that IL-36γ-secreting CAR T cells had increased proliferation and cytokine release and were associated with long-term remission in a mouse model of lymphoma and enhanced antitumor activity in a xenograft model. These results suggest that engineering CAR T-cells to secrete IL-36γ may improve responses to CAR T-cell therapy.
Additional research on blood cancers
As highlighted by the presentations discussed above, research is key to understanding mechanisms of treatment resistance in lymphoma and developing new immunotherapeutic strategies that may improve responses.
To learn about additional advances in blood cancer research, be sure to check out:
- A collection of recent lymphoma research papers from the AACR journals
- A study on CAR T-cell therapy for multiple myeloma and a related commentary in Blood Cancer Discovery, a journal of the AACR
- A conversation between CAR T-cell therapy pioneer Carl June, MD, and Blood Cancer Discovery. The conversation was also featured on this blog.



