New Dimensions in Cancer Biology: Updated Hallmarks of Cancer Published
The new year brings a new chapter in the holy book of cancer biology with the publication in the AACR journal Cancer Discovery of Hallmarks of Cancer: New Dimensions, an update to the “Hallmarks of Cancer” series. The latest edition was saluted with great enthusiasm by the scientific community as the new piece of an iconic saga, with many scientists taking to Twitter to share their excitement about seeing their field of study acknowledged among the fundamentals of cancer biology.
The original article of the seminal series was published in 2000 by Robert Weinberg, PhD, FAACR, from the Whitehead Institute for Biomedical Research and the Massachusetts Institute of Technology, and Douglas Hanahan, PhD, FAACR, from EPFL, the Swiss Federal Institute of Technology in Lausanne. The authors, both cancer research pioneers, organized state-of-the-art knowledge on cancer into a logical framework that recapitulated the extraordinary complexity of the disease in a small set of fundamental traits shared by most, if not all, human cancers. In addition, they introduced the concept of “enabling characteristics,” or means that enable premalignant cells to acquire the six hallmarks of cancer.
This landmark review soon became an essential resource for cancer researchers, with tens of thousands of citations, providing a comprehensive foundation for understanding and studying cancer biology. To account for new discoveries and progress in the field, the authors provided a first update in 2011, adding two emerging hallmarks and a new enabling characteristic.
In “Hallmarks of Cancer: New Dimensions,” Hanahan further revisited the list, proposing one new emerging hallmark and two additional enabling characteristics.
Read on to learn more about the hallmarks of cancer, how they were expanded over time, and the latest additions.
The original hallmarks (2000)
Writing about the overwhelming complexity of the scientific literature on cancer in 2000, Weinberg and Hanahan forecasted that, rather than adding more information in a chaotic fashion, research in the next quarter century would bring a conceptual shift towards a more logical approach to decipher such complexity “in terms of a small number of underlying principles.” The original “Hallmarks of Cancer” review was the authors’ effort and contribution to this shift, leading to the enumeration of six core “rules” that orchestrate the multistep process of the transformation of normal cells into malignant cells:
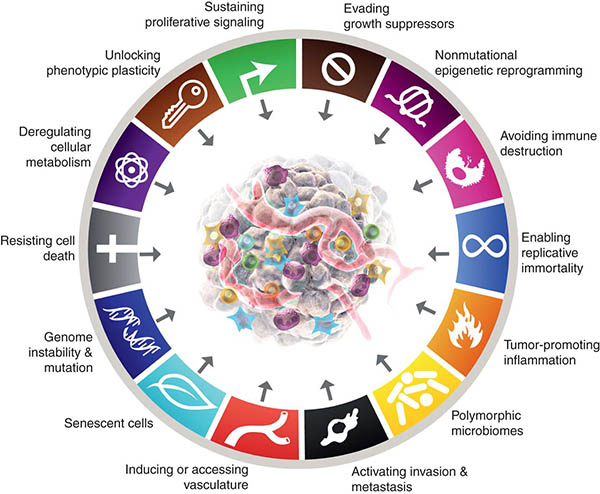
- Self-sufficiency in growth signals. While normal cells depend on external growth signals for proliferation, cancer cells can generate most of the growth signals by themselves, greatly reducing, or eliminating, their dependence on external stimuli. A corollary to this observation was a new view of cancer as a complex tissue in which malignant cells co-opt the surrounding normal cells to provide the necessary growth signals, serving as active collaborators, rather than passive bystanders.
- Insensitivity to growth suppressive signals. Multiple antiproliferative signals maintain the homeostasis in normal tissues, pushing cells out of the cell cycle and into a temporary quiescent state, or sending them into their terminal, post-mitotic differentiation state. Transformed cells evade these antiproliferative signals by subverting the mechanisms that control cell cycle progression—for example, by disrupting the pRb pathway, and overexpressing growth-stimulating factors such as c-myc.
- Ability to evade programmed cell death. Apoptosis is a major anticancer barrier, as becoming immortal is another way through which cancer cells continue to expand in number. Further, the authors proposed that the redundancy in cell death mechanisms could be exploited for therapeutic purposes.
- Enabling replicative immortality. In order for cancer to grow, malignant cells have to proliferate indefinitely. While normal cells possess a limited proliferative potential and will stop dividing at some point if cultured in vitro, cancer cells have lost that restrain mechanism, governed by telomere shortening. To become immortal, malignant cells rely on the telomerase enzyme to maintain the length of their telomeres above a critical threshold that allows them to go on dividing.
- Sustained angiogenesis. The growing tumor tissue has increased oxygen and nutrient needs and, to keep expanding, it needs to trigger the formation of new vasculature by releasing pro-angiogenic signals. At the time the authors codified this feature, it had been established that tumors go through an “angiogenic switch” that allows them to grow from microscopic to macroscopic lesions.
- Tissue invasion and metastasis. Metastasis is the cause of the vast majority of cancer deaths. The ability to invade, settle in, and grow in distant tissues is therefore one of the main features of cancer and relies on modifications in the cancer cell interactions with their surrounding environment through e-cadherin, integrins, and other adhesion molecules, and the production of matrix-degrading proteases.
The acquisition of multiple mutations through the loss of one or more mechanisms designated to protecting genome integrity was presented by the authors as an enabling characteristic that allows cancer cells to reach the six “biological endpoints” illustrated above.
Weinberg and Hanahan described the six capabilities acquired by cancer cells as the successful breaching of just as many anticancer defense mechanisms wired into our cells, and suggested these characteristics were shared by the more than 100 distinct types of cancer known at the time. Thus, the hallmarks of cancer provided a few unifying concepts toward which future cancer research could gravitate.
“The next generation” (2011)
In 2011, Weinberg and Hanahan published an update discussing the progress made over the preceding decade in the knowledge about the six original hallmarks. They also incorporated two emerging hallmarks:
- Reprogramming energy metabolism. While normal cells use oxygen to process glucose and produce energy, malignant cells can switch to aerobic glycolysis even in the presence of oxygen (what is known as the Warburg effect). Though this mechanism is less efficient, it is faster and originates several intermediate precursors used by cancer cells as building blocks to make proteins, DNA, and lipids to support their fast proliferation. Other cancer cells can use lactate as their main energy source.
- Evading immune destruction. Hanahan and Weinberg discussed evidence supporting the central role played by the immune system as a barrier to tumorigenesis, including studies in mouse models demonstrating that carcinogen-induced tumors developed and grew more rapidly in immunodeficient mice, especially if they lacked cytotoxic and helper T cells or natural killer cells, and observations that human tumors with high immune infiltration had better prognosis.
The 2011 edition also identified tumor-promoting inflammation as a new enabling characteristic. While immune infiltrates were historically considered a sign of the immune system reacting against the tumor, at the time the second review was published, the tumor-promoting effect of certain inflammatory cells had become clear. The authors discussed how inflammation favors multiple hallmark capabilities by providing growth, survival, and proangiogenic factors, and releasing chemicals, such as reactive oxygen species, that can cause additional mutations in the nearby cancer cells.
The review also contains a paragraph on the tumor microenvironment, which in the previous decade had become the subject of extensive research showing that, when studying the biology of a tumor, one needs to consider both the cancer cells and the microenvironment they construct around them.
“New Dimensions:” Expanding the Frontiers of Cancer Biology (2022)
Ten years later, Hanahan goes back to the hallmarks once more, recognizing the great progress made in the study of cancer through big data, and reaffirming the impact of the hallmarks of cancer in conceptualizing the new discoveries and “helping to distill this complexity into an increasingly logical science.” In the latest article, the two hallmarks added as emerging in 2011 were definitively incorporated as core hallmarks, as research in the past 10 years has largely confirmed the importance of metabolic reprogramming and avoiding immune destruction in cancer. In addition, Hanahan proposed an additional emerging hallmark:
- Phenotypic plasticity and disrupted differentiation. Terminal differentiation in normal cells is associated with a permanent proliferation arrest, and increasing evidence indicates that malignant cells evade differentiation and unlock what is known as phenotypic plasticity to continue to grow. In other words, they can change their identity into something that is more inclined to proliferate. This can happen in different ways: Cells that are approaching full differentiation can de-differentiate back to a progenitor-like state; neoplastic cells originating from an undifferentiated progenitor cell can halt the differentiation process and remain in that partially differentiated, progenitor-like state; and cells that were committed to a certain differentiation phenotype can switch developmental programs, or transdifferentiate, acquiring traits that are not associated with their cell of origin. Hanahan notes that, as it is true for other hallmark capabilities, cellular plasticity is not a “novel invention” of cancer cells, but rather a malignant twist on existing mechanisms that some normal cells can activate to repair and regenerate normal tissues.
The new article also highlights two new enabling characteristics:
- Non-mutational epigenetic reprogramming. Global changes in the epigenetic landscape are indeed recognized as a common feature of many cancers. Reproducing what happens during normal embryogenesis and development, cancer cells can reprogram a large number or gene-regulation networks to alter gene expression and favor the acquisition of hallmark capabilities.
- The microbiome. Our body is colonized by a vast array of microorganisms—nearly 40 trillion cells—that live in and on us. Their profound contribution to human health and disease is now appreciated. For example, researchers have found that some of these microorganisms can exert protective or deleterious effects on cancer development, progression, and response to therapy.
The new edition acknowledged the importance of senescent cells as instrumental components of the tumor microenvironment. While in the 2000 edition the authors discussed senescence as a possible anticancer barrier, they did not rule out the possibility of it being an artifact of cell culture that did not represent a real cell phenotype in vivo. More than two decades later, the role of cellular senescence in tissue homeostasis and cancer is well recognized, and significant morphological and metabolic features associated with it have been uncovered. Research has also shown how, in certain contexts, senescent cells can stimulate tumor development and malignant progression. Therefore, Hanahan proposed that senescent cells should be included as significant components of the tumor microenvironment.
In the concluding remarks, Hanahan explains how, while some of the hallmarks are now well validated, the newest features added as emerging hallmarks are meant to serve as “trial balloons” to stimulate debate within the cancer research community and inspire new investigations that will keep refining our understanding of cancer biology.
On to the next decade of discoveries.




Cancers are a complicated disease that causes rapid multiplication and growth of specific cells of the body. It can lead to cancer or neoplasm, an unusual mass of tissues.
Oncology is a medical discipline dealing with the diagnosis and management of cancer. If left untreated, it can progress into serious conditions endangering the life of the patients. Understanding disease at the cellular level can upgrade. The accuracy of disease diagnosis, management, and prevention of cancers. Additionally, a solid understanding of cancer with the assistance of disease tests from patients encourages clinical interpretations to forestall, treat and alleviate disease and related intricacies.
https://centralbiohub.de/biospecimens/cancer-samples