Editors’ Picks, September 2025: Cervical Cancer Screening, Immunotherapy Breakthroughs, Metabolic Vulnerabilities, and More
September may signal the end of summer, but in cancer research, the heat is still on. From cracking the code on why some leukemias outsmart venetoclax to detecting cancers years before symptoms appear, September’s lineup is packed with discoveries that could rewrite the playbook on prevention, detection, and treatment. You’ll find a precision methylation test that enhances cervical cancer screening, a dual DGK inhibitor that supercharges immunotherapy, and a first-in-class midkine inhibitor taking aim at triple-negative breast cancer. Add in adaptive therapy strategies, biomarker-driven clinical trials, and a deep dive into cancer metabolism, and you’ve got a collection of studies that prove innovation never takes a season off.
Keep reading for the abstract of each highlighted study and follow the links for the full-text articles, which are freely available for a limited time.
Journal: Blood Cancer Discovery
Resistance to venetoclax (VEN)-based therapy in acute myeloid leukemia (AML) includes genetic (i.e., mutations in N/KRAS, FLT3-ITD, TP53) and phenotypic (i.e., monocytic differentiation) features. Whether monocytic differentiation contributes to clinical VEN resistance secondary to a genetic bias remains unknown. This multimodal, multicenter, international analysis, inclusive of 678 patients, comprehensively characterized the prognostic role of monocytic differentiation in patients with AML treated with hypomethylating agents combined with VEN. AML genetics and monocytic differentiation (HR = 1.89; 95% confidence interval, 1.35–2.66; P < 0.001) in NPM1 wild-type cases correlated with an increased risk of death, clustering of centralized quantitative multiparameter flow cytometry data, evaluation of RNA sequencing-derived AML maturation stage, and single-cell proteogenomics linked driver mutations with AML phenotype and antiapoptotic gene expression. This comprehensive analysis of AML genetics, phenotype, and antiapoptotic protein expression highlights the complementary role these factors impart following VEN-based therapy.
Significance: AML with monocytic differentiation often occurs in the context of co-occurring mutations within signaling pathways. In certain AML subgroups (such as NPM1 wild-type and signaling pathway gene–mutated), a monocytic phenotype is associated with decreased overall survival following VEN-based therapy.
This article was highlighted and a related commentary was published in the September issue.
Journal: Cancer Discovery
Detection of Cancers Three Years prior to Diagnosis Using Plasma Cell-Free DNA
To explore how early cancers can be detected prior to clinical signs or symptoms, we assessed prospectively collected serial plasma samples from the Atherosclerosis Risk in Communities study, including 26 participants diagnosed with cancer and 26 matched controls. At the index time point, 8 of these 52 participants scored positively with a multicancer early detection test. All eight participants were diagnosed with cancer within 4 months after blood collection. In six of these eight participants, we were able to assess an earlier plasma sample collected 3.1 to 3.5 years prior to clinical diagnosis. In four of these six participants, the same mutations detected by the multicancer early detection test could be identified but at 8.6- to 79-fold lower mutant allele fractions. These results demonstrate that it is possible to detect ctDNA more than 3 years prior to clinical diagnosis and provide benchmark sensitivities required for this purpose.
Significance: Earlier detection is a promising strategy to reduce cancer mortality. For cancers of all stages, therapies are more effective with a lower disease burden. In this study, we demonstrate that ctDNA is detectable 3 years or more before cancer diagnosis and provide estimates for the sensitivity required to achieve such very early detection.
This article was featured on the cover and highlighted in the September issue, which also included a related commentary.
Journal: Cancer Epidemiology, Biomarkers and Prevention
Background: Proteomics could enhance our understanding of endometrial carcinogenesis. However, addressing confounding in traditional observational studies remains challenging, especially given the strong impact of adiposity on the plasma proteome and endometrial cancer risk.
Methods: Using Mendelian randomization (MR) and colocalization analyses, we examined the causal association between 2,751 unique proteins from the UK Biobank (N proteins = 2,031; N = 52,363) and deCODE (N proteins = 1,667; N = 35,559) with endometrial cancer risk [overall (N cases = 12,270; N controls = 46,126), endometrioid (N cases = 8,758), and nonendometrioid (N cases = 1,230)]. We performed enrichment analyses to explore pathways overrepresented among plasma proteins in endometrioid and nonendometrioid cancer subtypes. We assessed whether circulating proteins mediated the effect of body mass index on endometrial cancer risk using uni- and multivariable MR.
Results: Twenty proteins were associated with endometrial cancer risk in MR and colocalization analyses. GSTO1-1 and SKAP1 were positively and MMP10 was negatively associated with overall and endometrioid endometrial cancer; DTYMK and ABO were positively and TSSC4 was negatively associated with overall endometrial cancer; IGF2R was positively associated with endometrioid cancer; and MAPK9 was positively and DNAJB14, IFI16, LCN2, and SCT were negatively associated with nonendometrioid endometrial cancer. Distinct pathways were overrepresented in endometrioid (e.g., platelet-derived growth factor signaling and PTEN gene regulation) and nonendometrioid (e.g., noncanonical NF-κB signaling) cancer subtypes. There was weak evidence of associated proteins mediating the relationship between body mass index and endometrial cancer risk.
Conclusions: We identified distinct plasma proteins and pathways associated with endometrioid and nonendometrioid endometrial cancer risk.
Impact: Prioritized proteins may support noninvasive methods to differentiate endometrial cancer subtypes.
This article was highlighted in the September issue.
Journal: Cancer Immunology Research
Diacylglycerol kinase α (DGKα) and DGKζ are lipid kinases that negatively regulate T-cell signaling through diacylglycerol metabolism, making them attractive targets for next-generation immunotherapy. In this study, we report the discovery and preclinical characterization of the clinical-stage DGKα and DGKζ lipid kinase inhibitor, BMS-986408. BMS-986408 binds to the accessory subdomain of the catalytic domain and inhibits DGKα/ζ through a mechanism of action that includes competitive inhibition for the diacylglycerol substrate, subcellular translocation to the plasma membrane, and proteosome-dependent degradation. DGKα/ζ inhibition markedly improved the therapeutic benefit of PD-1 therapy by unleashing T-cell responses in the tumor while also amplifying the priming and expansion of tumor-reactive T cells in tumor-draining lymph nodes. Simultaneous inhibition of both DGKα and DGKζ was required to maximize combination benefit with PD-1 therapy. Furthermore, we observed in non–small cell lung cancer (NSCLC) patient samples that DGKα and DGKζ were broadly expressed in tumor-infiltrated T cells and that combination therapy invigorated a robust cytokine response in organotypic tumors derived from patients with NSCLC, supporting the clinical evaluation of this combination in patients with NSCLC. BMS-986408 also markedly improved CD19-targeted CAR T-cell therapy efficacy by overcoming hypofunctionality, insufficient expansion, and lack of costimulatory ligands. BMS-986408 represents a critical step toward evaluating the broad immunotherapy potential of DGKα/ζ inhibitors in patients with cancer.
This article was featured on the cover of the September issue.
Journal: Cancer Prevention Research
Cervical cancer is one of the most common cancers in women. Despite progress in prevention and success in early detection through cytologic screening and human papillomavirus (HPV) detection, there remains a challenge in triaging women appropriately to colposcopy and biopsy. We sought to validate the CervicalMethDx test, a precision DNA methylation classifier for cervical cancer detection, as a reflex test in women with HPV-positive samples. A blinded retrospective study was performed on well-characterized samples in PreservCyt media from a large referral clinical laboratory in the United States. DNA methylation was assessed in three gene promoters (ZNF516, FKBP6, and INTS1) and a control gene (β-actin) by quantitative real-time methylation-specific PCR (qMSP) analysis, using machine learning algorithms. We compared DNA methylation levels in HPV-positive patients presenting with lesions in the Pap test and cervical intraepithelial neoplasia grade 2 (CIN2) or CIN3 histologic diagnosis with DNA methylation levels in HPV-positive patients with lesions in the Pap test but no intraepithelial lesion or malignancy. The CervicalMethDx test correctly classified 95% of the CIN2 samples (n = 210), with 91% sensitivity, 100% specificity, and an area under the ROC curve (AUC) of 0.96, and 94% of CIN3 samples (n = 141), with 90% sensitivity, 100% specificity, and an AUC of 0.96. Moreover, the CervicalMethDx test correctly classified 94% of combined CIN2/CIN3 samples (n = 351), with 93% sensitivity, 97% specificity, and an AUC of 0.96. CervicalMethDx demonstrated strong discriminatory power for identifying CIN2/CIN3 risk and may complement current triage strategies for colposcopy referral. Prospective, population-based studies, including those in low-resource settings, are needed for further evaluation.
Prevention Relevance: The CervicalMethDx test integrates DNA methylation analysis and machine learning to improve early detection of high-grade cervical lesions (high-grade squamous intraepithelial lesions), offering a noninvasive, cost-effective screening tool. Enhanced risk stratification and overtreatment reduction expand equitable access to precision prevention programs. Further validation will clarify CervicalMethDx’s alignment with global cervical cancer prevention strategies.
This article was featured on the cover of the September issue.
Journal: Cancer Research (September 1 Issue)
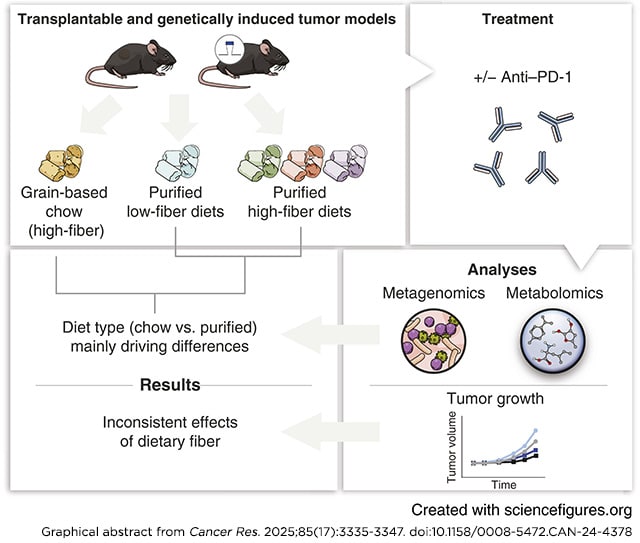
Immune checkpoint blockade (ICB) has transformed cancer treatment, but success rates remain limited. Recent research suggests that dietary fiber enhances ICB efficacy through microbiome-dependent mechanisms. However, prior studies in mice compared grain-based chow (high fiber) with low–fiber-purified diet, but these diets also differed in other dimensions, including phytochemicals. Therefore, further work is needed to establish the robustness of the effect of fiber on ICB across cancer types and dietary contexts. In this study, we investigated gut microbiome composition, metabolite levels, and ICB activity in mice fed with grain-based chow or purified diets with differing quantities of isolated fibers (cellulose and inulin). Compared with dietary fiber content, consumption of chow versus purified diet had a greater effect on the gut microbiome and a much stronger impact on the metabolome. Studies in multiple tumor models revealed that fiber has a weaker impact on ICB (anti–PD-1) efficacy than previously reported. Although diet affected ICB in some models, the effect was not directionally consistent. None of the models tested displayed the pattern expected if fiber controlled ICB efficacy: strong efficacy in both chow and high–fiber-purified diet but low efficacy in low–fiber-purified diet. Thus, dietary fiber seems to have limited or inconsistent effects on ICB efficacy in mouse models, and other dietary factors that correlate with fiber intake may underlie clinical correlations between fiber consumption and immunotherapy efficacy.
Significance: Clinical associations between high-fiber diets and improved immunotherapy efficacy may be driven by dietary factors correlated with fiber intake rather than fiber itself, which could impact dietary recommendations for patients undergoing immunotherapy.
Journal: Cancer Research (September 15 Issue)
Drug resistance results in poor outcomes for patients with cancer. Adaptive therapy is a potential strategy to address drug resistance that exploits competitive interactions between sensitive and resistant subclones. In this study, we showed that adapting carboplatin dose according to tumor response (adaptive therapy) significantly prolonged survival of murine ovarian cancer models compared with standard carboplatin dosing, without increasing mean daily drug dose or toxicity. Platinum-resistant ovarian cancer cells exhibited diminished fitness when drug was absent in vitro and in vivo, which caused selective decline of resistant populations due to reduced proliferation and increased apoptosis. Conversely, fitter, sensitive cells regrew when drug was withdrawn. Using a bioinformatics pipeline that exploits copy number changes to quantify the emergence of treatment resistance, analysis of cell‐free DNA obtained longitudinally from patients with ovarian cancer during treatment showed subclonal selection through therapy, and measurements of resistant population growth correlated strongly with disease burden. These preclinical findings pave the way for future clinical testing of personalized adaptive therapy regimens tailored to the evolution of carboplatin resistance in individual patients with ovarian cancer.
Significance: Carboplatin adaptive therapy improves treatment efficacy without increasing daily dose due to reduced fitness of drug-resistant populations, which can be tracked using cfDNA and could direct adaptive therapy in future clinical trials.
A related commentary was published in the September 2025 issue.
Journal: Clinical Cancer Research (September 1 Issue)
Purpose: Angiosarcomas (AS) are rare vascular sarcomas. Secondary AS (sAS) arise from DNA-damaging factors such as radiotherapy and UV radiation (UV-AS) or due to chronic lymphedema. The prognosis for advanced AS is poor, with limited treatment options. Immune checkpoint inhibition is not approved for AS, but high intratumoral T-cell density and frequent mutations in sAS may support efficacy.
Patients and Methods: This prospective, single-arm, multicenter phase II trial assessed the efficacy and safety of cemiplimab (350 mg, intravenously every 3 weeks) in patients with locally advanced or metastatic sAS using a Simon’s two-stage design. The primary outcome was the best overall response rate within 24 weeks of treatment. Secondary outcomes included time to response, duration of response, progression-free survival, overall survival, and predictive biomarkers for treatment response.
Results: Eighteen patients (12 with AS from radiotherapy, 3 with UV-AS, and 3 with AS due to chronic lymphedema) were treated with cemiplimab. The best overall response rate was 27.8% (4 partial responses, 1 complete response), with a time to response of 2.6 months and a duration of response of 6.9 months. The median progression-free survival was 3.7 months, and the median overall survival was 13.1 months. Grade ≥3 immune-related adverse events occurred in 33.3% of patients. High tumor mutational burden was observed in three patients with UV-AS, two of whom showed a response. High intratumoral CD3+ (P = 0.019), CD4 (P = 0.046), CD8+ (P = 0.026), and FoxP3+ (P = 0.026) T-cell densities; low platelet-to-lymphocyte ratio (P = 0.026); and Colidextribacter abundance were associated with response.
Conclusions: Cemiplimab shows promising effectivity in sAS and warrants further investigation. Promising predictive blood and tissue biomarkers were identified, indicating potential for improved patient selection.
This article was featured on the cover of the September 1 issue.
Journal: Clinical Cancer Research (September 15 Issue)
Purpose: Neuroendocrine prostate cancer (NEPC) is an aggressive form of prostate cancer with poor prognosis and limited treatment options. As NEPC aberrantly expresses delta-like ligand 3 (DLL3), the activity of tarlatamab, a bispecific T-cell engager that directs cytotoxic T cells to DLL3-positive (DLL3+) cells, was evaluated in the DeLLpro-300 study (NCT04702737).
Patients and Methods: This was a phase 1b, open-label study evaluating tarlatamab monotherapy in patients with metastatic de novo or treatment-emergent NEPC defined by histologic, genomic, or IHC criteria. Tarlatamab was administered intravenously every 2 weeks at a dose of 100 mg with a 1-mg step dose. The primary objective was safety, and a secondary objective was objective response rate (ORR) per RECIST v.1.1; DLL3 expression was retrospectively assessed by IHC.
Results: Forty patients were enrolled (DLL3+ tumors, n = 18; DLL3− tumors, n = 14; and DLL3 unknown tumors, n = 8). The most common treatment-related adverse events were cytokine release syndrome (82.5%), dysgeusia (42.5%), and decreased appetite (40.0%). Cytokine release syndrome was predominantly of low grade (grade 1/2/3/4+, 62.5%/15%/5%/0%), occurred exclusively in cycle 1, and was transient in duration (median duration, 3 days). The ORR was 10.5% [95% confidence interval (CI), 2.9–24.8]; the median duration of response was 7.3 months in the overall cohort. Patients with DLL3+ tumors (vs. patients with DLL3−/DLL3 unknown tumors) achieved a higher ORR [22.2% (95% CI, 6.4–47.6) vs. 0% (95% CI, 0–15.4)] and radiographic progression-free survival rate at 6 months [27.7% (95% CI, 8.7–50.9) vs. 0%].
Conclusions: The DeLLpro-300 study provides preliminary evidence for the safety and antitumor activity of tarlatamab in DLL3+ NEPC.
Journal: Molecular Cancer Research
TRAP1, the mitochondrial isoform of HSP90, has emerged as a key regulator of cancer cell metabolism, yet the mechanisms by which it rewires nutrient utilization remain poorly understood. We previously reported that TRAP1 loss increases glutamine (Gln) dependency of mitochondrial respiration following glucose (Glc) withdrawal. In this study, we investigate how TRAP1 deletion impacts Glc metabolism and the mechanisms enabling Gln retention to support mitochondrial respiration via reductive carboxylation and the oxidative TCA cycle. TRAP1 knockout (KO) in bladder and prostate cancer cells recapitulates the carbon source–specific metabolic rewiring previously observed. Stable isotope tracing reveals that although Glc oxidation remains functional, TRAP1 KO reduces overall Glc uptake and its contribution to glycolysis and the pentose phosphate pathway. This effect is consistent across multiple cell lines. Concurrently, TRAP1-deficient cells exhibit increased Gln retention and reliance, potentially due to downregulation of the cystine/glutamate antiporter SLC7A11/xCT. Supporting this, xCT overexpression reduces Gln-dependent respiration in TRAP1 KO cells. qPCR and proteasome inhibition assays suggest that xCT is regulated posttranslationally via protein stability. Notably, xCT suppression does not trigger ferroptosis, indicating a selective adaptation rather than induction of cell death. Together, our findings suggest that TRAP1 loss decreases Glc uptake while preserving its metabolic fate, promoting Gln conservation through xCT downregulation to maintain mitochondrial respiration without inducing ferroptosis.
Implications: These results reveal a TRAP1-dependent mechanism of metabolic rewiring in cancer cells and identify xCT-mediated Gln conservation as a key adaptive response, underscoring TRAP1 as a potential metabolic vulnerability and therapeutic target in tumors with altered nutrient utilization.
This article was highlighted in the September issue.
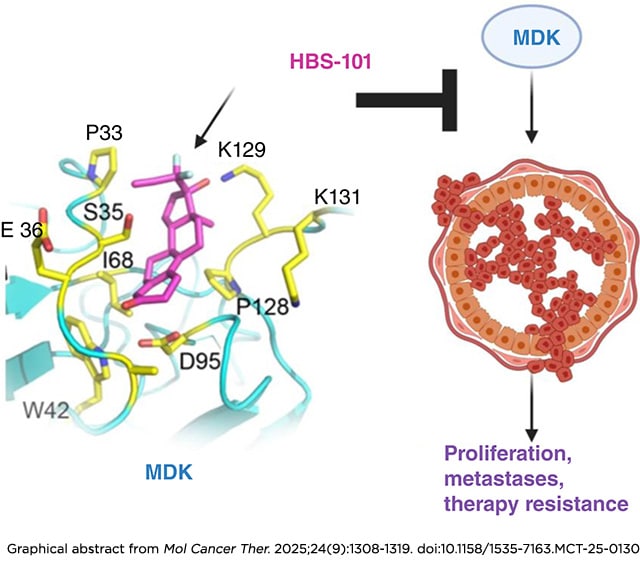
Journal: Molecular Cancer Therapeutics
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer with a poor clinical outcome. There is a dire need for the development of new targeted therapies for TNBC. Midkine (MDK), a multifunctional cytokine/growth factor, functions as an oncoprotein, and its expression is elevated in various cancers. The absence of small-molecule inhibitors targeting MDK represents a significant knowledge gap for translation. In this study, we identified HBS-101 as a potent MDK inhibitor with high specificity. Our modeling studies revealed that the interaction of HBS-101 with MDK is primarily driven by hydrophobic forces, and this interaction disrupted MDK’s binding to its endogenous receptors. Microscale thermophoresis, cellular thermal shift assay, and biotin pull-down studies confirmed the direct interaction of HBS-101 with MDK. Therapeutically, HBS-101 treatment significantly reduced cell viability (IC50 0.3–2.8 µmol/L), clonogenic survival, invasiveness, and increased apoptosis. The underlying mechanism of HBS-101 involves suppression of the Akt/mTOR, STAT3, and NF-κB pathways. Importantly, HBS-101 exhibits distinct pharmacologic advantages, including oral bioavailability, blood-brain barrier penetration, and in vivo stability. Histologically, doses of up to 10 mg/kg showed no observable organ toxicity and had no effect on the mice’s body weight. Dose range studies identified 5 mg/kg as the minimal effective dose, achieving more than a 50% tumor reduction. HBS-101 treatment led to a significant reduction in the growth of xenograft tumors derived from patients with TNBC in vivo and markedly reduced TNBC brain metastatic tumor growth and prolonged mice survival. Collectively, our studies identified a first-in-class MDK inhibitor, HBS-101, that can be used to treat MDK-driven cancers.
This article was featured on the cover and highlighted in the September issue.
Journal: Cancer Research Communications
Current response evaluation methods have accuracy limitations. Monitoring with a ctDNA methylation-based tumor fraction (TF) may provide a more accurate assessment of tumor burden across solid tumors. In this study, we evaluate a tissue-free, methylation-based TF and explore the association with treatment outcomes in RADIOHEAD, a cohort of 1,070 patients with solid tumors receiving standard-of-care immune checkpoint inhibition (ICI) regimens, with blood samples collected prospectively for retrospective analysis. A total of 1,997 baseline and serial on-treatment plasma samples from 627 patients with stage IV cancer were analyzed with an analytically validated next-generation sequencing methylation-based ctDNA assay (Guardant Reveal). The primary outcome measure was real-world progression-free survival (rwPFS). Secondary outcomes included real-world overall survival (rwOS) and the lead time between nonmolecular response (nMR) to rwPFS event. Patients with any decrease in TF while receiving ICI had superior outcomes. Patients with ≥80% decrease in TF at two timepoints or TF below the limit of quantification had longer rwPFS and rwOS than those with <80% decrease [nMR; rwPFS HR, 0.24 (95% confidence interval, 0.19–0.32) P < 0.005; rwOS HR, 0.28 (95% confidence interval, 0.21–0.38) P < 0.005]. nMR was detected prior to clinical progression in 209 patients with a median lead time of 3.03 months. Among 627 patients with stage IV cancer receiving standard-of-care ICI, monitoring with methylation-based TF identified patients who have significantly longer rwPFS and rwOS. Changes in TF on ICI can provide additional response data earlier than imaging alone and support the potential of serial monitoring to inform treatment decisions.
Significance: This study found that early decreases in tumor DNA levels in the blood are linked to longer survival in patients with cancer treated with immunotherapy. These findings support the use of blood-based monitoring to help predict treatment response and improve decision-making in real-world cancer care.