Revising the Dosing of a Cancer Immunotherapeutic
The FDA has approved an alternative dosing schedule for the immune checkpoint inhibitor pembrolizumab. On April 28, 2020, the...


The FDA has approved an alternative dosing schedule for the immune checkpoint inhibitor pembrolizumab. On April 28, 2020, the...
The FDA approved a new molecularly targeted therapeutic for certain patients with lung cancer driven by a mutation in...
The FDA has approved a type of molecularly targeted therapeutic called an antibody-drug conjugate for the treatment of metastatic...
The FDA has approved a new molecularly targeted therapeutic for treating certain patients with advanced or metastatic HER2-positive breast...
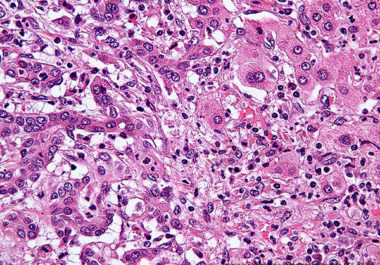
The first molecularly targeted therapeutic for use in the treatment of bile duct cancer has been approved by the...
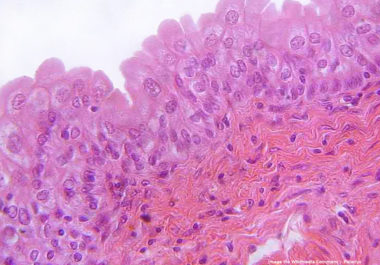
The FDA has approved a chemotherapy gel for treating certain patients with cancer of the upper urinary tract. he...

The FDA has approved a molecularly targeted therapeutic for treating certain patients with neurofibromatosis type 1 who have inoperable...
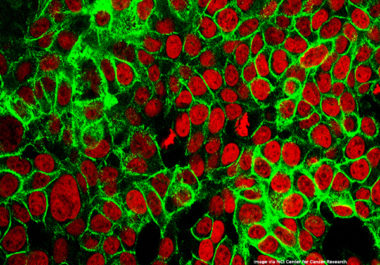
The FDA has approved a new combination of molecularly targeted therapeutics for treating metastatic colorectal cancer with a BRAF...
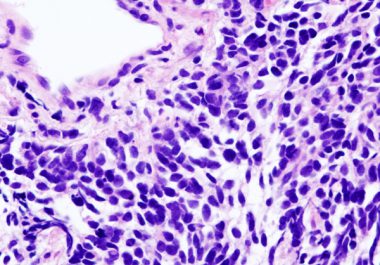
The FDA has approved a new combination of immunotherapy and chemotherapy as an initial treatment for extensive-stage small cell...
The FDA approved using a combination of nivolumab and ipilimumab, two immune checkpoint inhibitors, to treat certain patients with...