A New Type of Immunotherapy for Melanoma
The U.S. Food and Drug Administration approval provides a new option for patients with the most deadly form of...

The U.S. Food and Drug Administration approval provides a new option for patients with the most deadly form of...
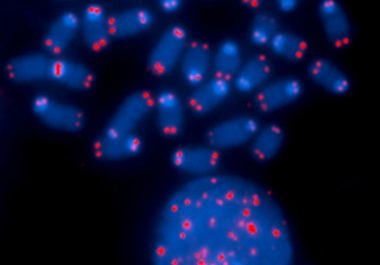
The U.S. Food and Drug Administration approval provides a new option for patients with liposarcoma and leiomyosarcoma that is...
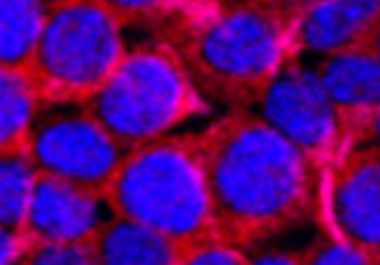
The U.S. Food and Drug Administration approval provides a new option for patients who face a poor prognosis Onivyde,...
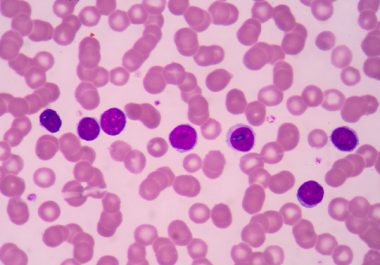
The U.S. Food and Drug Administration approval provides a new option for patients whose disease has worsened after treatment...

The FDA-approved therapeutic is the first to target the T790M EGFR mutation that frequently causes resistance to other EGFR-targeted...
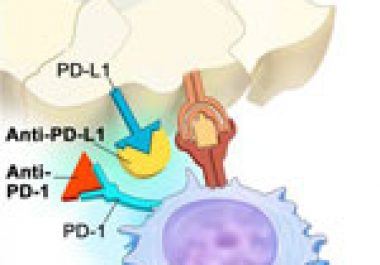
The U.S. Food and Drug Administration approval expands the use of the immunotherapeutic pembrolizumab to certain patients with the...
Approved immunotherapeutics release different brakes on the immune system. A new combination of cancer immunotherapeutics – ipilimumab (Yervoy) and...
The U.S. Food and Drug Administration approval provides a new option for patients whose disease is no longer responding...

About nine out of 10 U.S. thyroid cancer diagnoses are differentiated thyroid cancers; the FDA approval provides a new...

Ibrutinib – a drug that targets the molecule that fuels the growth of B cells in Waldenstrom macroglobulinemia and...