First Immunotherapy-Companion Diagnostic Combo for Advanced Lung Cancer
The U.S. Food and Drug Administration approval expands the use of the immunotherapeutic pembrolizumab to certain patients with the most common form of lung cancer.
The U.S. Food and Drug Administration (FDA) has approved an expansion in the use of pembrolizumab (Keytruda) to include patients who have advanced non-small cell lung cancer (NSCLC) that has progressed after other treatments and whose tumors test positive for the protein PD-L1.
The FDA simultaneously approved a companion diagnostic test called PD-L1 IHC 22C3 pharmDx to identify the patients whose tumors expressed the PD-L1 protein.
NSCLC is the most common type of lung cancer. Progress against advanced lung cancer is urgently needed because the majority of patients newly diagnosed with lung cancer already have advanced disease and face a poor prognosis; the five-year survival rate for patients with advanced lung cancer is just 4 percent.
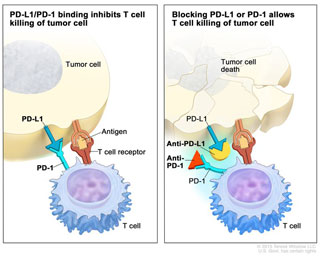
Pembrolizumab works by blocking the PD-1/PD-L1 pathway that cancer cells sometimes engage in order to apply “brakes” on cancer-fighting T cells, preventing the T cells from doing their job. Blocking the PD-1/PD-L1 pathway releases the brakes on T cells and enables them to fight cancer cells.
Pembrolizumab was approved by the FDA for treating advanced melanoma in September 2014. It is the second PD-1 inhibitor to be approved for NSCLC, the first being nivolumab, which was approved for treating patients with the squamous form of NSCLC in March 2015.
The FDA’s approval of pembrolizumab for advanced NSCLC was based on results from a subgroup of patients enrolled in the KEYNOTE-001 phase I clinical trial. Pembrolizumab shrank tumors in 41 percent of patients who had NSCLC that had progressed following chemotherapy or targeted therapy and whose tumors tested positive for PD-L1 using the PD-L1 IHC 22C3 pharmDx test, and the treatment effect lasted between 2.1 and 9.1 months.
Early results from this trial were presented at the AACR Annual Meeting 2015 and simultaneously published in the New England Journal of Medicine.
The FDA approval was rendered on Oct. 2, 2015.
