February 28, 2020: The Week in Cancer News
Researchers find a possible explanation for the link between certain gut bacteria and colorectal cancer and other cancer news of the week from Cancer Today magazine staff.


Researchers find a possible explanation for the link between certain gut bacteria and colorectal cancer and other cancer news of the week from Cancer Today magazine staff.
The FDA has expanded the use of the molecularly targeted therapeutic neratinib to include the treatment of certain patients with advanced or metastatic breast cancer
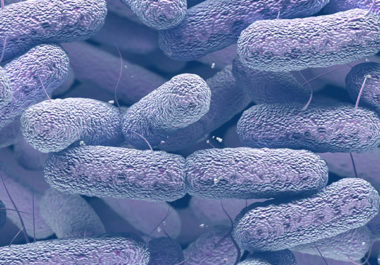
“There’s a clear connection between cancer and the gut microbiome,” began Matthew Redinbo, PhD, of the University of North Carolina at Chapel Hill, at the opening keynote address during the recent AACR special conference The Microbiome, Viruses, and Cancer, held in Orlando. Redinbo, a professor of chemistry, biochemistry, and microbiology, noted that there is an “intimate chemical relationship” linking specific microbiota with cancer progression, and that certain bacteria can also modulate patient’s responses to cancer treatment, representing an untapped area of drug discovery.

Tracking health problems in young cancer survivors and other cancer news of the week from Cancer Today magazine staff.
Circulating tumor DNA (ctDNA) was first detected over 30 years ago, but its potential uses for cancer diagnosis and treatment are only recently being extensively explored. An emerging method known as liquid biopsy examines ctDNA, cell-free DNA (cfDNA), or circulating tumor cells in blood plasma samples to learn about the patient’s cancer without needing to resect tissue from the tumor itself, as is typically required in traditional diagnostic and staging methods. Liquid biopsy could, therefore, provide a minimally invasive technique to detect and characterize cancer and to monitor its response to treatment.
February is National Cancer Prevention Month. We recently had the opportunity of speaking with American Association for Cancer Research (AACR) Past President William Hait, MD, PhD, a leading expert in cancer prevention and interception. Hait, who is a Fellow of the AACR Academy and the current AACR treasurer, forecasted several key areas of advancement in this field. In this excerpt from our previous post, Hait shares his predictions for cancer prevention and interception research in 2020.

A study illuminates the alcohol consumption habits of cancer survivors.

Today is International Day of Women and Girls in Science, a United Nations initiative aimed at encouraging full and equal participation in sciences for women and girls around the world. The American Association for Cancer Research (AACR) is proud to have a powerful roster of female leaders in science. Cancer Research Catalyst posts in the past two years have captured their messages of empowerment and encouragement.

Due to the high cost of care and cost-sharing, many of the more than 16.9 million survivors in the United States face significant financial challenges. Research-driven progress against cancer has created a growing population...
Medical oncologist and lung cancer expert Roy S. Herbst, MD, PhD, discusses early data on drugs that target a mutated form of the KRAS protein